Question
I need help figuring out these problems. if you could please help that would be lovely and will you please write clearly. Thank you Problem
I need help figuring out these problems. if you could please help that would be lovely and will you please write clearly. Thank you
Problem #1
A series of standards were made as described in Table 1. A 0.2240 g sample of ore was digested in 25 mL of concentrated nitric acid for 10 minutes. The resulting solution (solution X1) was diluted with water to a total volume of 100.0 mL. A 20.00 mL aliquot of solution X1 was mixed with potassium ferrocyanide to produce a blue color from the complex formed with Fe3+ ion and this second solution (X2) was diluted to a total volume of 50.00 mL. The absorbance of solution X2 was 0.517. What is the percent iron in the ore, with error?
Table 1. Standards for the visible spectroscopic analysis of iron. [Fe3+]stock = 104.8 ppm. Total volume = 25.00 mL. reagent = 1 M K4Fe(CN)6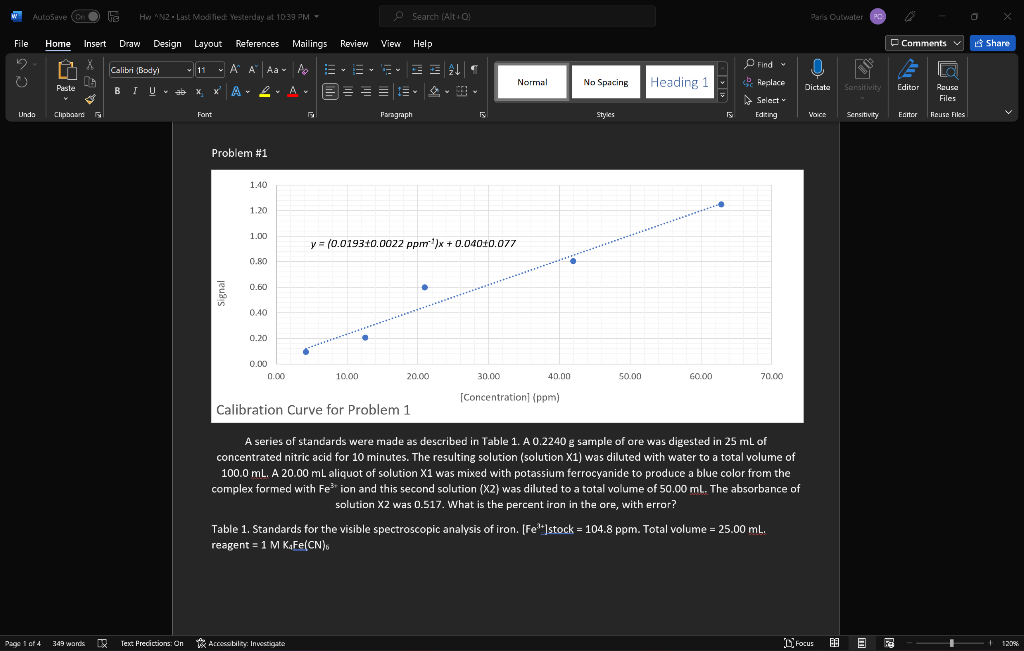
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


