Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. 4. 6. 7. 10. 11- PH 2.13. 2.17 15. 2.21 2.34 2.46 2.68 2.96 8. 9. 11.76 4-78 11.33 11.59 12. 11-72 13.
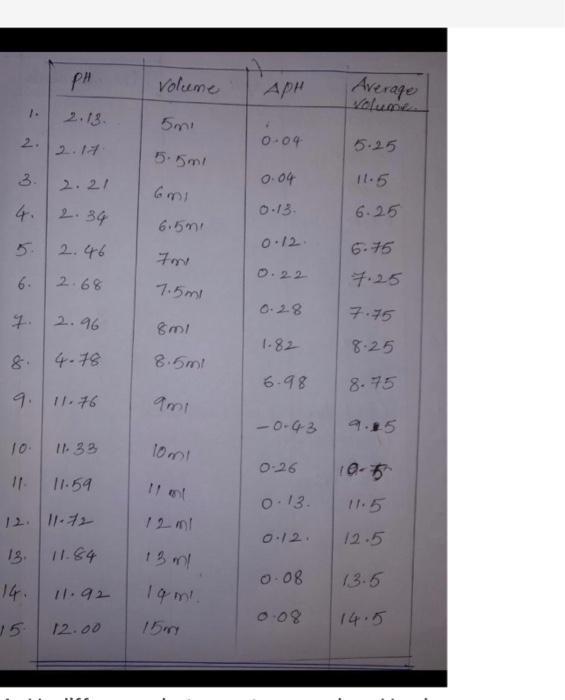
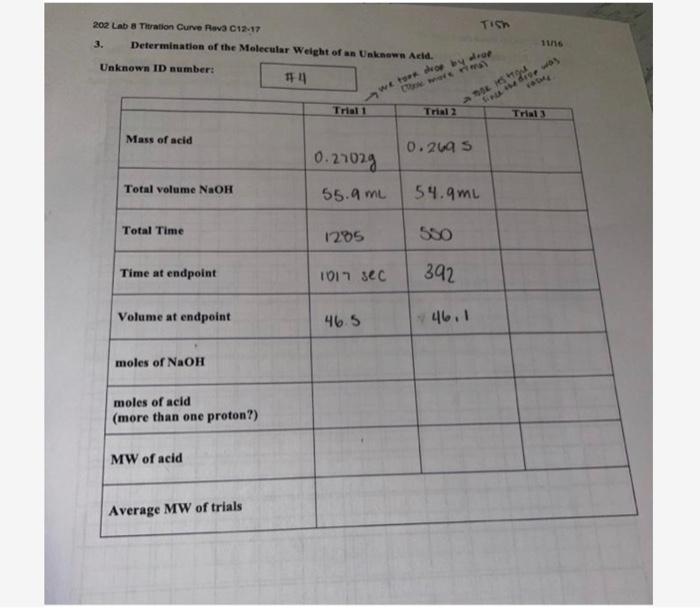
2. 4. 6. 7. 10. 11- PH 2.13. 2.17 15. 2.21 2.34 2.46 2.68 2.96 8. 9. 11.76 4-78 11.33 11.59 12. 11-72 13. 11.84 11.92 12.00 Volume 5mi 5.5ml 6m1 6.5m1 701 7.5ml 8ml 8.5ml 9m1 10mt 13 ml 19 m. 15m APH 7 0.04 0.04 0.13. 0.12. 0.22 0.28 1.82 6-98 -0-43 0-26 0.13. 0.12. 0.08 Average volume 5.25 11.5 6.25 6-75 7.25 7.75 8.25 8.75 9.25 10-5 11-5 12.5 13.5 14.5 202 Lab 8 Titration Curve Rev3 C12-17 Determination of the Molecular Weight of an Unknown Acid. Unknown ID number: Mass of acid Total volume NaOH Total Time Time at endpoint Volume at endpoint moles of NaOH moles of acid (more than one proton?) MW of acid Average MW of trials #41 my we took drop by drop Trial 1 0.27029 55.9 ML 1285 1017 sec 46.5 Trial 2 0.2695 550 Tish & TOOK its trg 392 54.9mL 46.1 11/16 Since the drop was Trial 3
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Solution Purpose Determine the precise concentration of NaOH by titrating the NaOH solution against ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


