Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help find the concentration of yhe unknown Cu sample. Thank you. Preparation of standard solutions. a. In preparation for the determination of Cu2
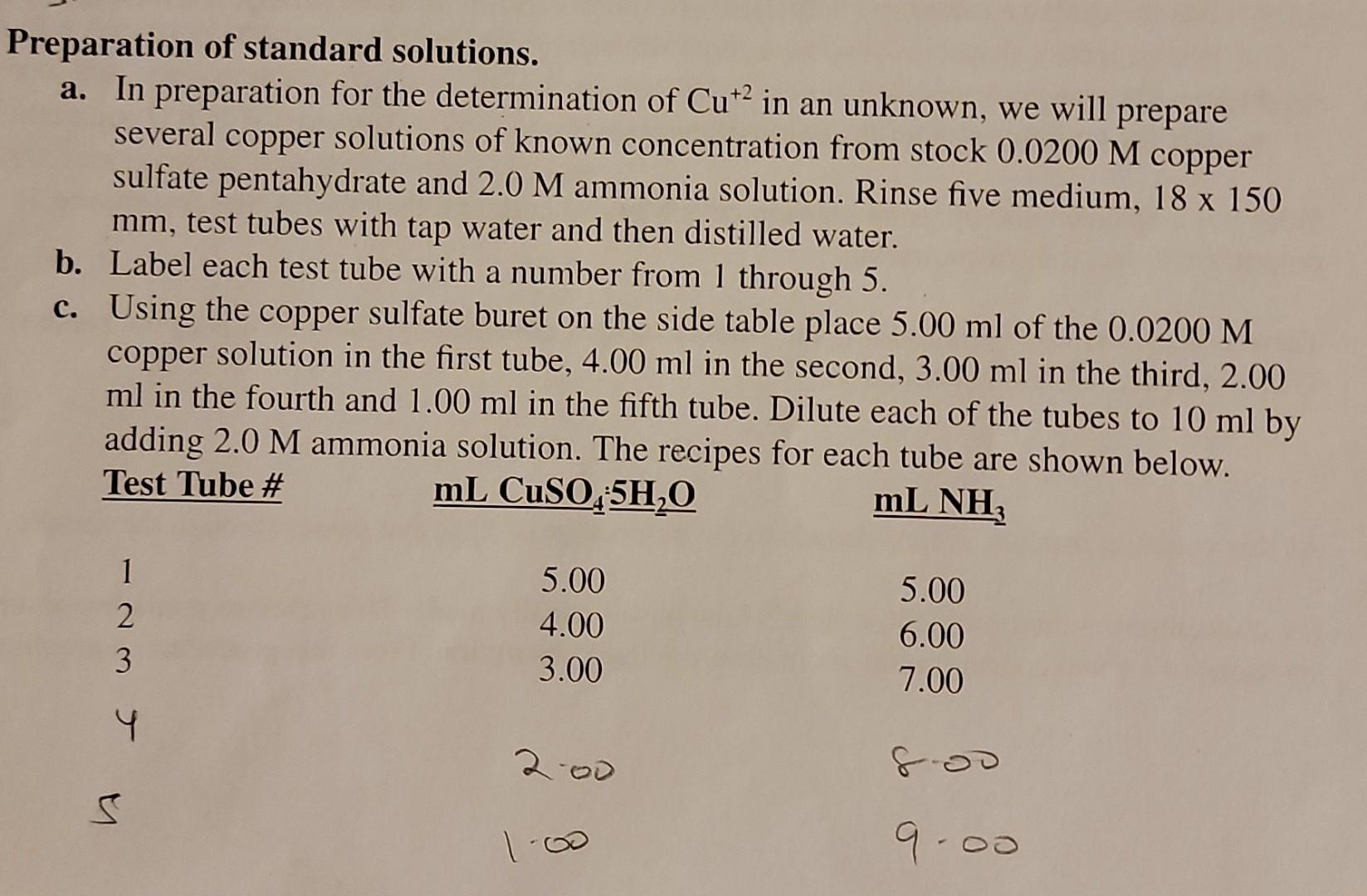

I need help find the concentration of yhe unknown Cu sample. Thank you.
Preparation of standard solutions. a. In preparation for the determination of Cu2 in an unknown, we will prepare several copper solutions of known concentration from stock 0.0200 M copper sulfate pentahydrate and 2.0 M ammonia solution. Rinse five medium, 18 x 150 mm, test tubes with tap water and then distilled water. b. Label each test tube with a number from 1 through 5. c. Using the copper sulfate buret on the side table place 5.00 ml of the 0.0200 M copper solution in the first tube, 4.00 ml in the second, 3.00 ml in the third, 2.00 ml in the fourth and 1.00 ml in the fifth tube. Dilute each of the tubes to 10 ml by adding 2.0 M ammonia solution. The recipes for each tube are shown below. Test Tube # mL CuSO,5H,0 mL NH 1 2 3 5.00 4.00 3.00 5.00 6.00 7.00 4 200 9.00 mivi= M2 V2 2. Concentration Calculations for the Five Test Tubes Tube #1 .02X5ml - .olm Tube #2 02 X 4 mL = .008mL Tube #3 -02X 3 me oobme 10 me Tube #4 .02x2 me 004 me tome Tube 75 .02X ImL =.002 mL 12 On Find the concentration of unknown Cu sample. (show Calculation) Preparation of standard solutions. a. In preparation for the determination of Cu2 in an unknown, we will prepare several copper solutions of known concentration from stock 0.0200 M copper sulfate pentahydrate and 2.0 M ammonia solution. Rinse five medium, 18 x 150 mm, test tubes with tap water and then distilled water. b. Label each test tube with a number from 1 through 5. c. Using the copper sulfate buret on the side table place 5.00 ml of the 0.0200 M copper solution in the first tube, 4.00 ml in the second, 3.00 ml in the third, 2.00 ml in the fourth and 1.00 ml in the fifth tube. Dilute each of the tubes to 10 ml by adding 2.0 M ammonia solution. The recipes for each tube are shown below. Test Tube # mL CuSO,5H,0 mL NH 1 2 3 5.00 4.00 3.00 5.00 6.00 7.00 4 200 9.00 mivi= M2 V2 2. Concentration Calculations for the Five Test Tubes Tube #1 .02X5ml - .olm Tube #2 02 X 4 mL = .008mL Tube #3 -02X 3 me oobme 10 me Tube #4 .02x2 me 004 me tome Tube 75 .02X ImL =.002 mL 12 On Find the concentration of unknown Cu sample. (show Calculation)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


