Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help for the three questions A buffer solution is made that is 0.328M in HF and 0.328M in NaF. (1) If Ka for
i need help for the three questions 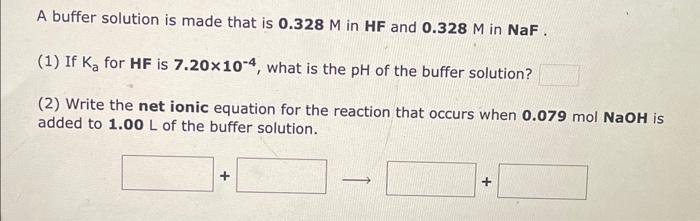


A buffer solution is made that is 0.328M in HF and 0.328M in NaF. (1) If Ka for HF is 7.20104, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.079molNaOH is added to 1.00L of the buffer solution. A buffer solution is made that is 0.354M in HNO2 and 0.354M in NaNO2. (1) If Ka for HNO2 is 4.50104, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.098molHNO3 is added to 1.00L of the buffer solution. Use H3O+instead of H+. A buffer solution is made that is 0.468M in CH3COOH and 0.468M in CH3COO. (1) If Ka for CH3COOH is 1.80105, what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.100molHBr is added to 1.00L of the buffer solution. Use H3O+instead of H+ 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


