Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help for the three questions For each of the salts on the left, match the salts on the right that can be compared
i need help for the three questions 
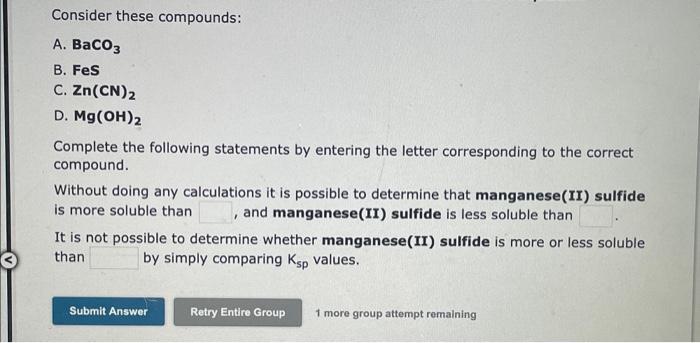

For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. cobalt(II) carbonate A. Cos 2. iron(II) hydroxide B. MnS C. Pb(OH)2 D. PbSO4 Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. cobalt(II)carbonateKsp=iron(II)hydroxideKsp= Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s. Consider these compounds: A. BaCO3 B. FeS C. Zn(CN)2 D. Mg(OH)2 Complete the following statements by entering the letter corresponding to the correct compound. Without doing any calculations it is possible to determine that manganese(II) sulfide is more soluble than , and manganese(II) sulfide is less soluble than It is not possible to determine whether manganese(II) sulfide is more or less soluble than by simply comparing Ksp values. 1 more group attempt remaining For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. barium fluoride A. CuCO3 2. calcium chromate B. Pb3(PO4)2 C. Mn(OH)2 D. Ag2CrO4 Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. bariumfluorideKsp=calciuKsp= calcium chromate Note: Multiply out any number and put it first in the K5p expression. Combine all exponents for s 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


