Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help The pH scale goes from 0.0 to 14.0. The lower the pH meosurement, the more aciclic a solution is the higher the
i need help 
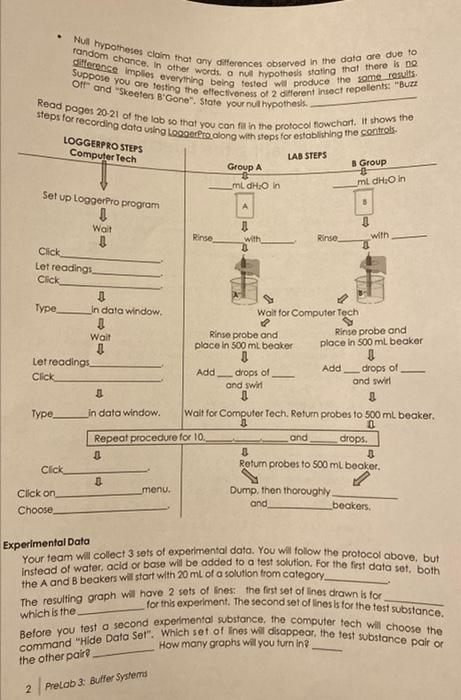
The pH scale goes from 0.0 to 14.0. The lower the pH meosurement, the more aciclic a solution is the higher the number, the more bosic a solution is. Lobel the arows below the scole os "more ocidic" or "more basic". What number is neutral on the pH scale? (Decimols counti) The higher the concentrotion of hydrogen lons - symbolized as [H], the more acidic a sclution is. Ar acld is an ionic molecule that roleases H in a water-based solution. causing an increase in [H+]. A bose is an lon/c molecule that relcoses an lon which removes H-from solution, resulting in a decrease in [H], Lobel the arrows over the scale as "decreasing [H+] or "increcsing [H] ". A bulfer system works to keep pH whin a nanow range, Buffer systems are critical in oll living celb/issues. It pH were to measurably shilt in a cell. what category of biological molecules would be most impocted, and what term refers to the resuling loss of their functional shapes? - Null hypotheses cloim that any differences observed in the data are due to OIt and "Skeeters B'Gone". State your nul hypothesk. Read poges 2021 of the lob so that you con fil in the protocol flowchart. It sthows the Experimental Date Your team will colect 3 sets of experimental data. You will follow the protocol above, but instead of water, acid or bose will be added to a test solution. For the first data set, both the A and B beokers will start with 20mL of a solution trom category, The resulting graph will have 2 sets of lines: the fist set of lines drown is for which is the for this experiment. The second set of lines is for the test substance. Before you test a second experimental substance. the computer tech will choose the Before you test a secondere Sel". Which sef of lines will disappear, the test substance pair or the other pair 2 Prelab 3. Buffer Systems The pH scale goes from 0.0 to 14.0. The lower the pH meosurement, the more aciclic a solution is the higher the number, the more bosic a solution is. Lobel the arows below the scole os "more ocidic" or "more basic". What number is neutral on the pH scale? (Decimols counti) The higher the concentrotion of hydrogen lons - symbolized as [H], the more acidic a sclution is. Ar acld is an ionic molecule that roleases H in a water-based solution. causing an increase in [H+]. A bose is an lon/c molecule that relcoses an lon which removes H-from solution, resulting in a decrease in [H], Lobel the arrows over the scale as "decreasing [H+] or "increcsing [H] ". A bulfer system works to keep pH whin a nanow range, Buffer systems are critical in oll living celb/issues. It pH were to measurably shilt in a cell. what category of biological molecules would be most impocted, and what term refers to the resuling loss of their functional shapes? - Null hypotheses cloim that any differences observed in the data are due to OIt and "Skeeters B'Gone". State your nul hypothesk. Read poges 2021 of the lob so that you con fil in the protocol flowchart. It sthows the Experimental Date Your team will colect 3 sets of experimental data. You will follow the protocol above, but instead of water, acid or bose will be added to a test solution. For the first data set, both the A and B beokers will start with 20mL of a solution trom category, The resulting graph will have 2 sets of lines: the fist set of lines drown is for which is the for this experiment. The second set of lines is for the test substance. Before you test a second experimental substance. the computer tech will choose the Before you test a secondere Sel". Which sef of lines will disappear, the test substance pair or the other pair 2 Prelab 3. Buffer Systems 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


