Question: I need help understanding . 2. Consider the following reaction: H2S(g)H2(g)+S(g)Kp=1.67107 PH2Sintial=3.00atm and PH2initial=0.00450atm. Determine PH2Scq,PH2eq, and PSeq
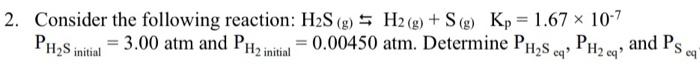
2. Consider the following reaction: H2S(g)H2(g)+S(g)Kp=1.67107 PH2Sintial=3.00atm and PH2initial=0.00450atm. Determine PH2Scq,PH2eq, and PSeq
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


