I need help with all of page 7 (Question 4&5) and completing the table (table 8) as well.
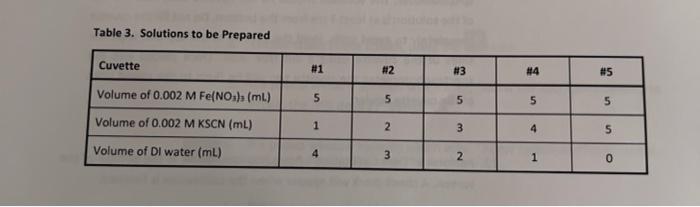
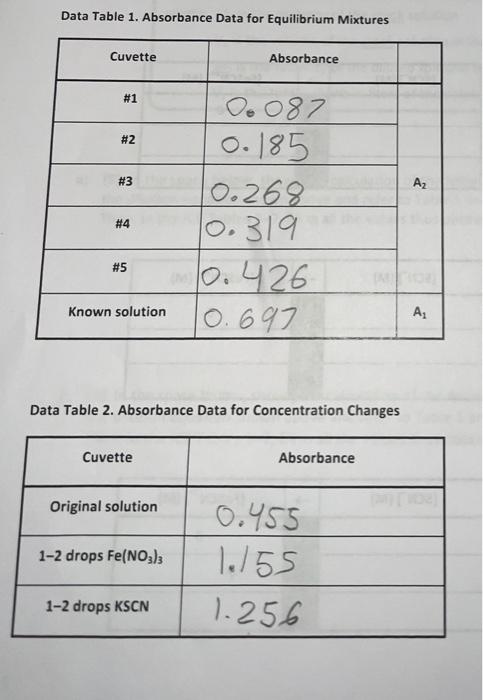
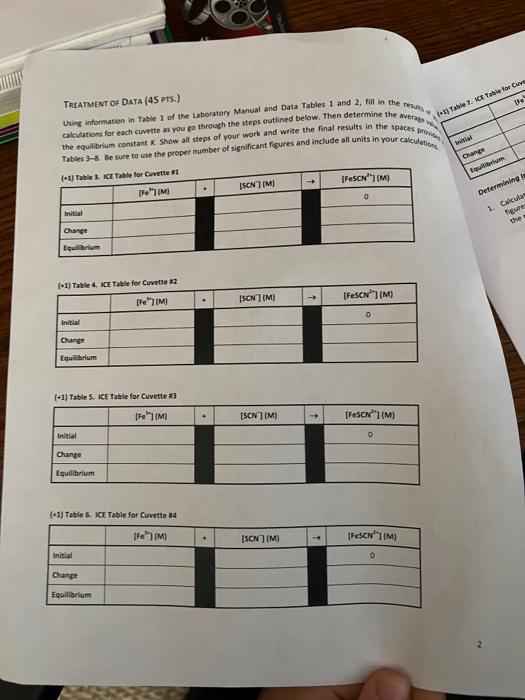
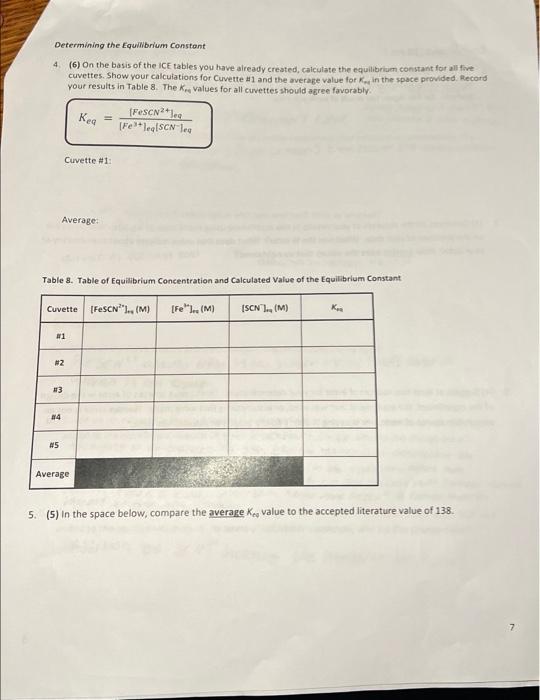
Table 3. Solutions to be prepared Cuvette #1 #2 2 #3 #4 #5 Volume of 0.002 M Fe(NOx) (ml) 5 5 5 5 5 Volume of 0.002 M KSCN (mL) 1 2 3 4 un Volume of Dl water (ml) 4 3 2 1 0 Data Table 1. Absorbance Data for Equilibrium Mixtures Cuvette Absorbance #1 #2 #3 Az Oo 087 0. 185 0.268 10.319 0.426 10.697 #4 #5 Known solution A. Data Table 2. Absorbance Data for Concentration Changes Cuvette Absorbance Original solution 1-2 drops Fe(NO3)3 0.455 1.155 1.256 1-2 drops KSCN flable. It Table for Cove TREATMENT OF DATA (45 PTS.) Using information in Table 1 of the Laboratory Manual and Data Tables 1 and 2, fill in the rest calculations for each cuvette as you go through the steps outlined below. Then determine the average the equilibrium constant Show all steps of your work and write the final results in the spaces Tables & Be sure to use the proper number of significant figures and include all units in your calculation Change Equilibrium (1) Table 3. ICE Table for Cuvette #1 [FOSCN (M) - ISON (M) IFM Determining Initial 1. Calculas figure Chan Equilibrium f1) Table 4. ICE Table for Cuvette 2 [Fe(M) ISON (M) [FeSON (M) 0 Initial Change Equilibrium (-1) Table 5. ICE Table for Cuvette #3 [Fe(M) (SCN (M) [Fec|(M) Initial 0 Change Equilibrium (-1) Table 5. ICE Table for Cuvette #4 re ISON (M) [FeSCOM) Initial 0 Change Equilibrium results of your of (-1) Table 7, ICE Table for Cuvettes FM Initial SCNUM IF SENIM 0 CILMAN Change Equilibrium Determining Initial Concentrations 1. Calculate the initial molarity for the two reactants fe and SCN (with the correct number of significant figures for each of the solutions that went into cuvettes w1S. Recall that the initial molar concentration of the product is equal to zero at the start of the reaction MV, M, V, where M is the molarity of the stock solution V is the volume of the stock solution in liters M is the molarity of the diluted solution that is in the cuvette V, is the total volume in liters of the solution that was made for the cuvette a) (3) In the space below, show your calculation of the initial molarity of fe. Fe, for cuvette 1. Use the dilution equation shown above and refer to Table 1 and the Procedure in the laboratory Manual Then, in the ICE Tables 3-7, fill in all the values thus obtained for the initial (Fe for each cuvette. b) (3) In the space below, show your calculation of the Initial molarity of SCN (SCN), for cuvette . Use the dilution equation shown above and refer to Table 1 and the Procedure in the Laboratory Manual. Then, in the ICE Tables 3-7, fill in all the values thus obtained for the initial (SCN7 for each cuvette. 19) Determining Change in concentrations 2 We will calculate the equilibrium concentration of resovand use this value to determine in the Change now of the table. We will use the Beer Lambert Lew that relates absorbance of colored species to the molar concentration of the coloured speciet The Beer Lambert equation is shown below. 130Calculate the concentration of the known solution of resc. This known solution was made in Part A Step in the experimental procedure in this procedural step, you measured out 2.00 mL of 0.00200 M NSC and then added 1000 ml of 0.200 Mre/No.1, and 8.00 mt oft water, for a total wolume of resulting solution of 20.00 mL in the space below, show your complete calculation for the concentration of tescil in the known solution (remember to use the correct number of significant figures Fel + SCN - FeSCN Hint for this cation recall that the NaSC Amiting reestant (there is a large excess of Fe(NOJ, to ensure that at the SC extentially converted into Fest base is complete when an equal number ces have combined at what is known as b) (6) Because absorbance for a given solution containing FeSCN is proportional to molar concentration of fesc" (as per Beer's Law), use a simple proportionality equation shown below to solve for Equilibrium concentration of resc in eachusette, Show your work for cuvette al in the secm below, and put in the Equilibrium values for frescu") in each cuvette into the respective it table AC where A is the measured absorbance (this is a unitless quantity the product is a constant composed of two separate constants called the molar absorptivity (e) and the thickness of the absorbing medium (b) C is the molar concentration of the absorbing species [FeSCN). [FeSCN20 ASTO where [Fes"), is the molar concentration of Festina particular cuvette A, is the measured absorbance for the solution in a particular cuvette [FeSCN2-re is the molar concentration of the known standard from Step 3/a) above Am is the measured absorbance of the known standard solution c)(3) Note that the concentrations that you just calculated are the equilibrium concentrations for Pesen" for each cuvette. Examine the last column in your ICE tables and notice that this concentration at represents the Change in Fec molar concentration, Now, enter in the Change in molarities of Fec, Feand SCN into the ice table for each cuvette (Tables 3-7). Be sure to indicate the sign of the individual values Determining Equilibrium Concentrations 3. (4) Next, calculate the Equilibrium molarities for fe and SCN from the values you have entered into the ICE table for each cuvette, using Initial molarity and the Change in molarity for these remaining species Show your calculations below for the Equilibrium molarities for Fe" and SCN for cuvette #1. Enter all the Equilibrium molarities for each cuvette in the ICE Table for the respective cuvette. This step will complete the ICE Table for each cuvette. (Fe") ISCN1 Question1 Volume of the solution Imi) Total Volume of SC Volume of 20 wolume solution (m) (ml) Initial concentrat Concentration ton of SCN- of Fe(M) M) Cuvette 10 1 2 3 4 3 2 1 66 868 10 0.001 0.001 0.001 0.001 0.001 0.0002 0.0004 0.0006 0.0008 0.001 4 5 Question (a) Initial concentration of Fellum effe centration of 10 Tatal me 5.00 0.002 M Initial conc. Of Fe3+= X 10.00 0.001 M Question(b) Sample calculation for Cuvettel: Initial concentratio of SCN -- folume of an af CN Tuta 1.00 Initial conc. Of SCN- X 10.00 0.002 M 0.0002 M Answer 1 of 1 Done 0.691 0.022 20 0:002 M AN x A A [resowe [ resonte len tresenology - [rescatalogo -0.0002 M: 10:08 = 2.49 X Cuck 2 [Pescartes)-0.0002 M Ce + = 0.97 = 0.265 093 10315 - 0.31 Cuvelike 3 + [fesent- 0.0002 MK 012) = 7.65 X-? Cuvette 4 - [Besentley = 0.0002 MX ( = 9:15* -SM Cuvches [tesen tel, -0.002M*(426 - 12.22% [fet [tes] - Cesently, [senten - [Sew), - Etes cwmy Cuvete [feer [SCN-Jeg 1 3 4 0.003 0.00014 0.000923 0-0003 0:000878 0.00015 0.00034 0.00 $2 0.006708 0.000814 [feseN +2] ez Rea [fe +3] ez [SEN Jer Cuvette Kea 2 3 4 S 145.93 161.59 159.30 142.33 158.52 Answer 1 of 1 Done ME Answer 1 of 1 Done Curtea diwalay (ret. - Pescar? of Pet 0.001M- -AX165) = 4.2x Esmolarity SON = salita - Pesan 0.0002 : - (-4*657 s 175x6m Taki-3_ewiele tolle Fest 0 [Fest) 1al 0.001 channe -2.4K Esime 9.75 [SOM 0.0002 -1-40215 1-75x6 a S-31X105 0 45 8 60x165 Tablice oble for cuvette #2 Fest SCN -> Folha 0.001 6-0004 Change -531x65 -S-315 has 9.476 2.41264 kes ICE bolle for cu ret SCM Sultas 0.001 0.0006 wa -7-69x155 -7.92185 eru 9.23x154 Tade Etalle foucette rest SON Tybal 0-001 0.000 ca - 1.15765 -4.155 clitius | 4.08 1-06 Tales Lable facute 35 F3t Sen Ded 0.001 0.001 Change - 1996 elf' S21X15 8x160 Fest +94157155 9.15.15 FESCAT 55 +19.92.5 19.116 Determining the Equilibrium Constant 4 (6) On the basis of the ICE tables you have already created, calculate the equilibrium constant for all five cuvettes. Show your calculations for Cuvette #1 and the average value for in the space provided. Record your results in Table 8. The values for all cuvettes should agree favorably Kea FeSCN2tles [Feleq[SCN les Cuvette #1: Average: Table 8. Table of Equilibrium Concentration and Calculated Value of the Equilibrium Constant Cuvette [Fesc"... (M) [Fe" ...(M ISCNI., (M) #1 #2 # 13 24 #5 # Average 5. (5) in the space below, compare the average Kre value to the accepted literature value of 138