Answered step by step
Verified Expert Solution
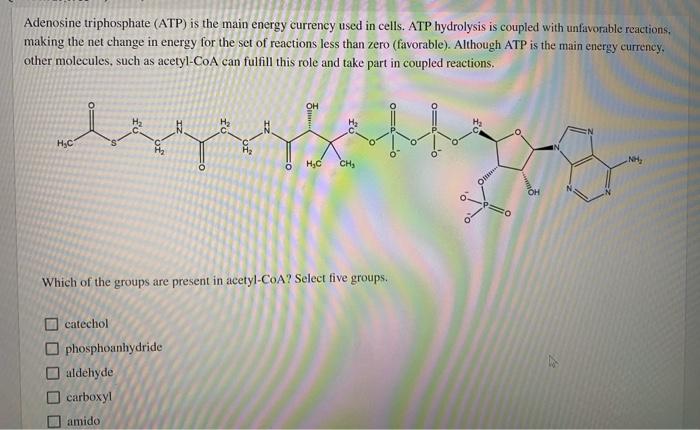
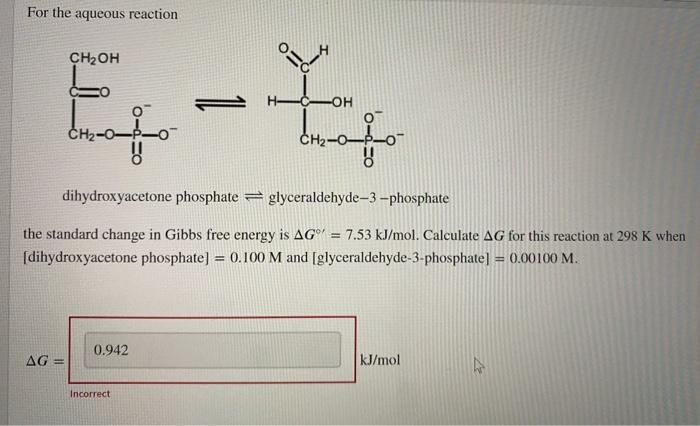
Question
1 Approved Answer
I need help with all of the photos posted. If you answer every question and its all correct, I will 100% upvote the answer. I
I need help with all of the photos posted. If you answer every question and its all correct, I will 100% upvote the answer. I will also share it with my friends who need the answer as well who will also upvote.
If not all questions are answered or are incorrect, I will downvote. I don't want to be mean, I'm just a broke college kid & don't have very many questions left. Photos 2 and 3 are the same question, I just couldn't fit all the choices in one photo.
Thank you so much in advance!


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


