Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help with all questions on all three pages 2. Explain the miscibility of ONE molecule of ethanol (CH,CH,OH) by drawing intermolecular forces (hydrogen
i need help with all questions on all three pages 
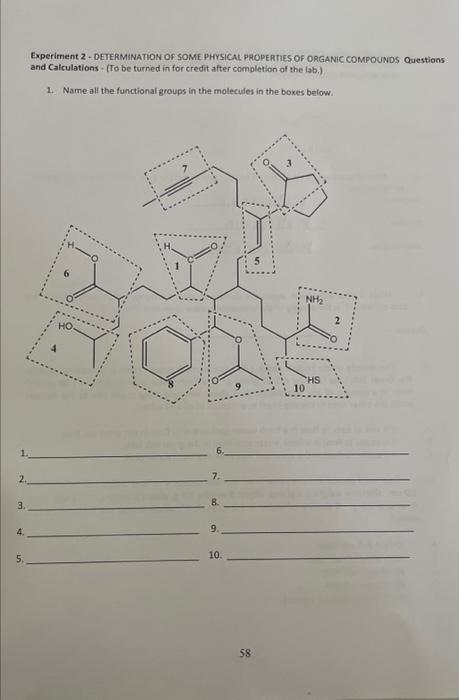
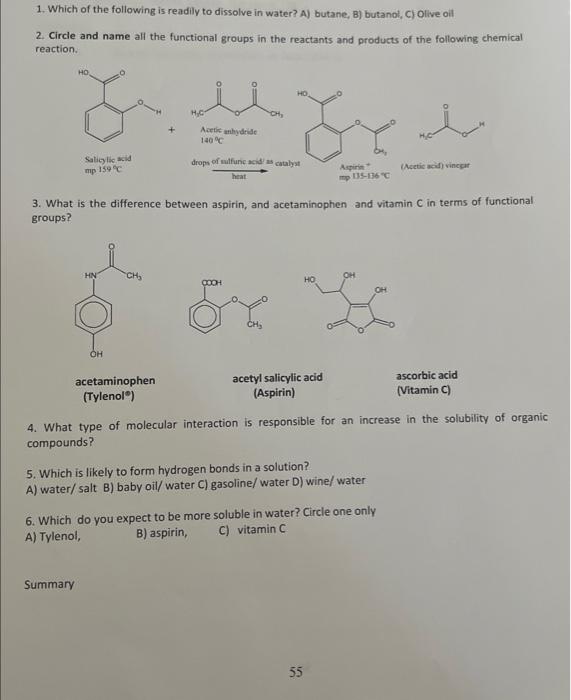
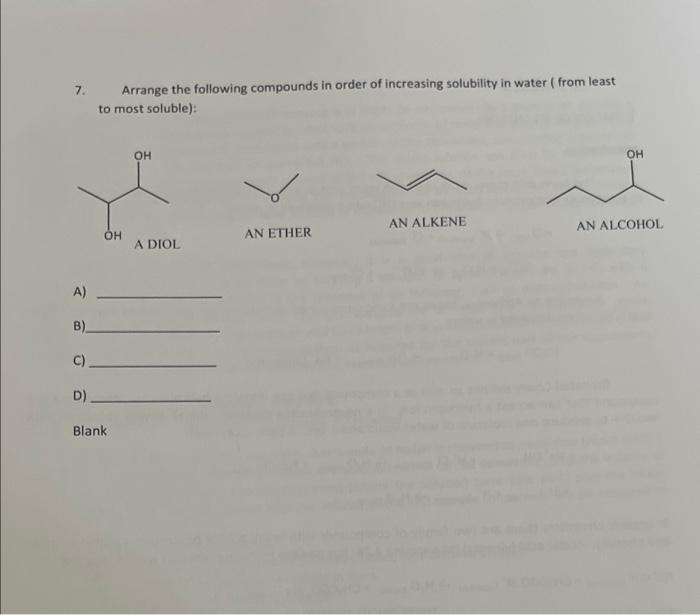
2. Explain the miscibility of ONE molecule of ethanol (CH,CH,OH) by drawing intermolecular forces (hydrogen bonding) with THREE water molecules. Do not forget the 2 lone pairs of electrons in oxygen. 3. After observation of your data on tables I and II, classify each substance as tydrophobic or thydrophilic. Decane aujdropaosionc. Ethanol hadrophisis. Amyl acetate husdrochoheic 4. What is your conciusion about the density of each substance when compared to the data on table l? is the density of the substance greater than that of water? Decane 5. The density of our body is about 1.03g/mL at room temperature. Is it easier to float in the Great Salt take (density =1.12g/mL ) or in a fresh water pool (density =1.00g/mL )? Hint: In a miature of any two things, think about why one thing might sink to the bottom while the other rises to the top (such as a mixture of oil and water). A. It is just as easy to float in a fresh water pool as it is to flost in the great salt lake. B. It is easier to float in a fresh water pool than in the great salt lake. C. It is easier to float in the great salt lake than in a fresh water pool. 6. Four solid objects are the same exact size and occupy a volume that is 2.0cm3.0cm 1.0cm. Object A welghs 6.5grams, object A weighs 5.1g. object C weighs 10.1g and object. D. weighs 9.3g. If density of water is 1.0grams/cm3, which one(s) will float in a tub of water? A. Only A \& B will float B. Only object D will float C. Allobjects will float D. All objects will sink E. None of the above is correct Experiment 2 - DETERMINATION OF SOME PHYSICAL PROPERTIES OF ORGANIC COMPOUNDS Questions and Calculations - (To be turned in for credit after completion of the lab.) 1. Name all the functional groups in the molecules in the boxes below. 1. 6, 2+ 7. 3. 8. 4. 9. 5. 10. 1. Which of the following is readily to dissolve in water? A) butane, B) butanol, C) Olive oil 2. Circle and name all the functional groups in the reactants and products of the following chemical reaction. Salicylic acid mip 159C heatdropsofmulfuncacidyascatalyst Aspirint 3. What is the difference between aspirin, and acetaminophen and vitamin C in terms of functional groups? acetaminophen acetyl salicylic acid ascorbic acid (Tylenolo) (Aspirin) (Vitamin C) 4. What type of molecular interaction is responsible for an increase in the solubility of organic compounds? 5. Which is likely to form hydrogen bonds in a solution? A) water/salt B) baby oil/ water C) gasoline/ water D) wine/ water 6. Which do you expect to be more soluble in water? Circle one only A) Tylenol, B) aspirin, C) vitamin C 7. Arrange the following compounds in order of increasing solubility in water ( from least to most soluble): AN ETHER AN ALKENE AN ALCOHOL A) B) C) D) 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


