Question
I need help with part C and D using the answers from part A and B. Information : Fritz Haber was awarded a Nobel Prize
I need help with part C and D using the answers from part A and B.
Information: Fritz Haber was awarded a Nobel Prize for the processes he invented in which nitrogen and hydrogen gases are combined to make ammonia (NH3NH3), a valuable chemical and a vital nutrient in modern agriculture. It is known that ammonia is 82.2%% nitrogen by mass.
Part A: If 27.3g of nitrogen with 5.91g of Hydrogen are combined in a closed system, what mass of Ammonia will be produced?
Answer: 33.2g NH3
Part B: What mass of Nitrogen is required to fully consume 11.5 g of hydrogen?
Answer: 53.2g N
Part C:
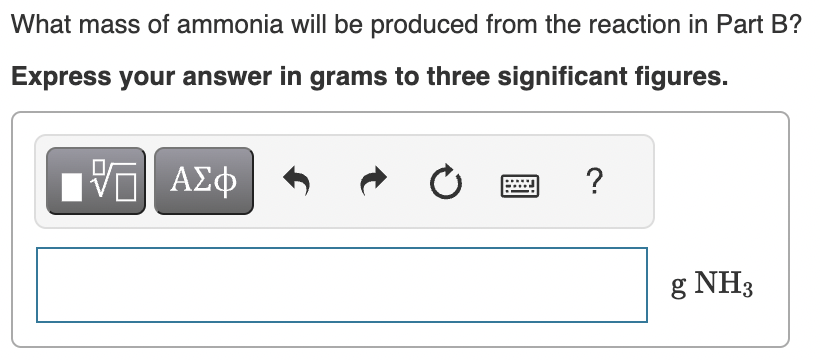
Part D:
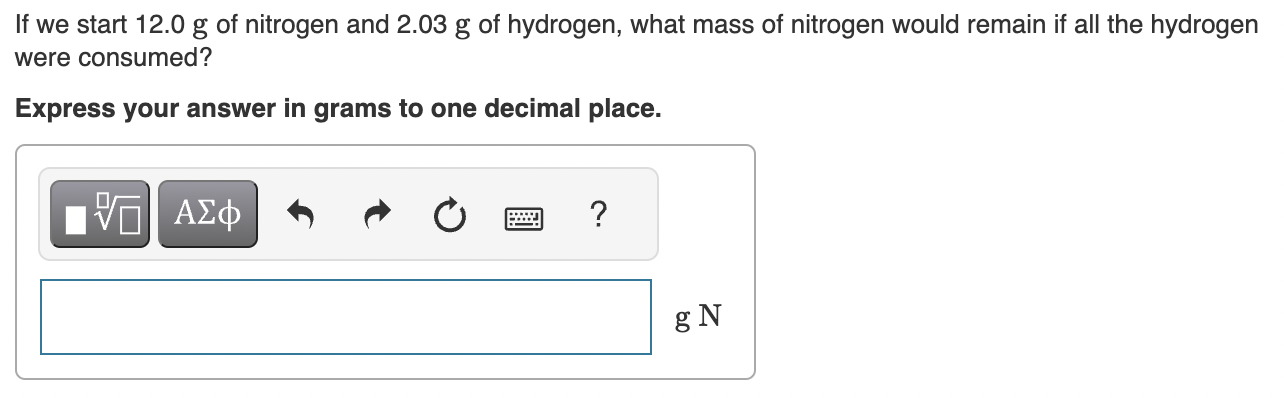
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


