Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need part b please (uses concentration from part a) i need part b MISSED THIS? Read Section 15.3 (Pages 637642 ). The following reaction
i need part b please (uses concentration from part a) 

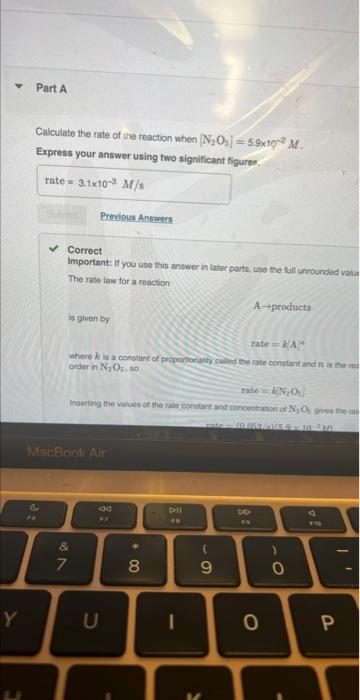
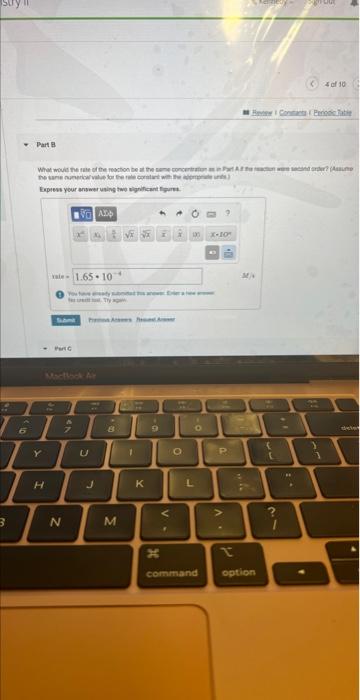
i need part b 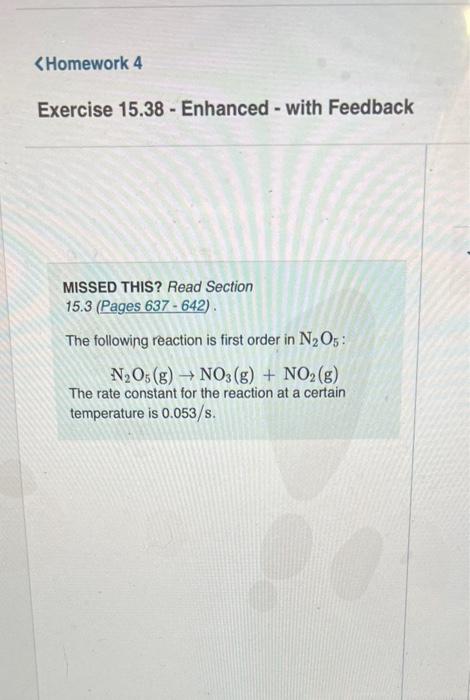
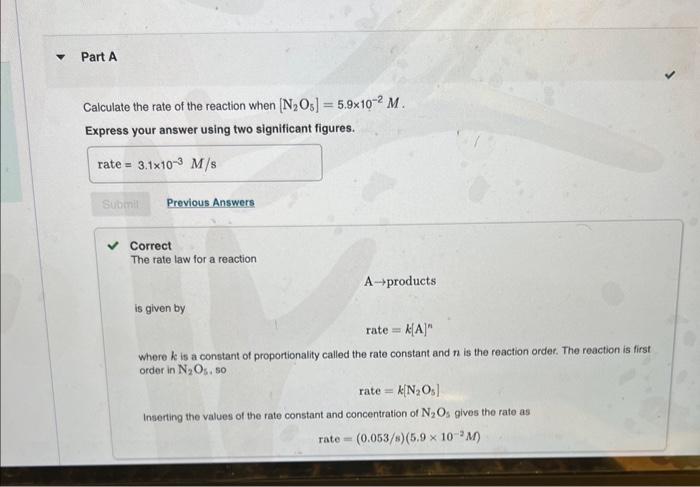
MISSED THIS? Read Section 15.3 (Pages 637642 ). The following reaction is first order in N2O5 : N2O5(g)NO3(g)+NO2(g) The rate constant for the reaction at a certain temperature is 0.053/s. Calculate the rate of the reaction when [N2O5]=5.9102M. Express your answer using two significant figures. Correct Important: If you use this answer in later parts, bse the fuil unrounded watu The rate law for a reaction is given by: A prodinctis rate=d.A Where k ia a constart of proporioniecr celied tme ratn conetant and n is the roth order in N2O5.60 Tate=KiN2O3} Inserting the values of the rate constart and conosntanon of N7O3 pyes the rat Eipiess your answar using two algnifesent figure. MISSED THIS? Read Section 15.3 (Pages 637-642) . The following reaction is first order in N2O5 : N2O5(g)NO3(g)+NO2(g) The rate constant for the reaction at a certain temperature is 0.053/s. Calculate the rate of the reaction when [N2O5]=5.9102M. Express your answer using two significant figures. Correct The rate law for a reaction Aproducts is given by rate=k[A]n where k is a constant of proportionality called the rate constant and n is the reaction order. The reaction is first order in N2O5, so rate=k[N2O5] Inserting the values of the rate constant and concentration of N2O3 gives the rate as rate=(0.053/s)(5.9102M) What would the rate of the reaction be at the same concentration as in Part A if the reaction were second order? (Assume the same numerical value for the rate constant with the appropriate units.) Express your answer using two significant figures. X Incorrect; Try Again 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


