Question
A polytropic process is a thermonic process that obeys the relation: pV constant The exponent a is known as the polytropic index, and it
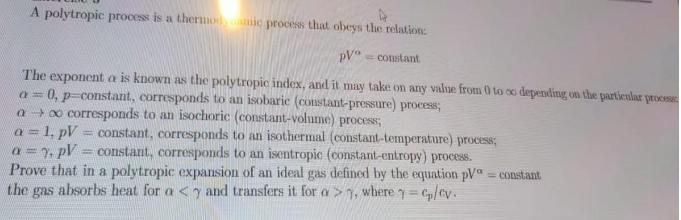
A polytropic process is a thermonic process that obeys the relation: pV constant The exponent a is known as the polytropic index, and it may take on any value from 0 to co depending on the particular process a= 0, p-constant, corresponds to an isobaric (constant-pressure) process; a o corresponds to an isochoric (constant-volume) process; a = 1, pV = constant, corresponds to an isothermal (constant-temperature) process; a = y, pV = constant, corresponds to an isentropic (constant-entropy) process. Prove that in a polytropic expansion of an ideal gas defined by the equation pV = constant the gas absorbs heat for a , where = cp/cv.
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Answer Molar heat capacity positive means it releases heat on expansion and negativ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Electric Machinery
Authors: Charles Kingsley, Jr, Stephen D. Umans
6th Edition
71230106, 9780073660097, 73660094, 978-0071230100
Students also viewed these Electrical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



