Question
9 mol P4010 reacts with 51 mol HO according to the equation below: P4010 + 6HO 4H3PO4 How many moles of H3PO4 form from
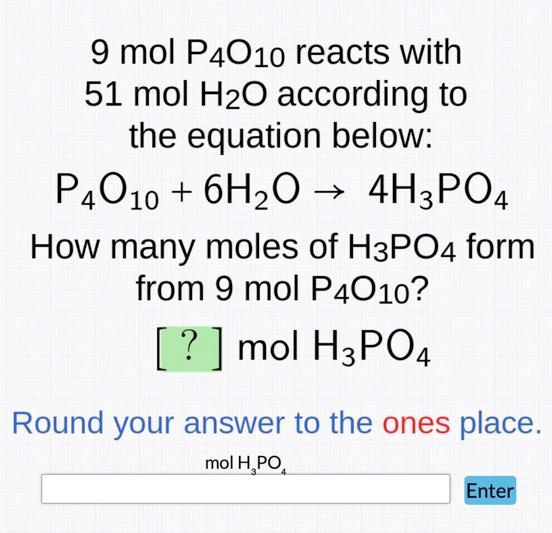
9 mol P4010 reacts with 51 mol HO according to the equation below: P4010 + 6HO 4H3PO4 How many moles of H3PO4 form from 9 mol P4010? [?] mol H3PO4 Round your answer to the ones place. mol HPO Enter
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The balanced chemical equation shows that 1 mole ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Algorithms
Authors: Thomas H. Cormen, Charles E. Leiserson, Ronald L. Rivest
3rd edition
978-0262033848
Students also viewed these Algorithms questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



