Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Spectroscopic Notation Consider the atom having the electron configuration 182s2p 3s3p. Part A Which of the following statements are correct? Check all that apply.
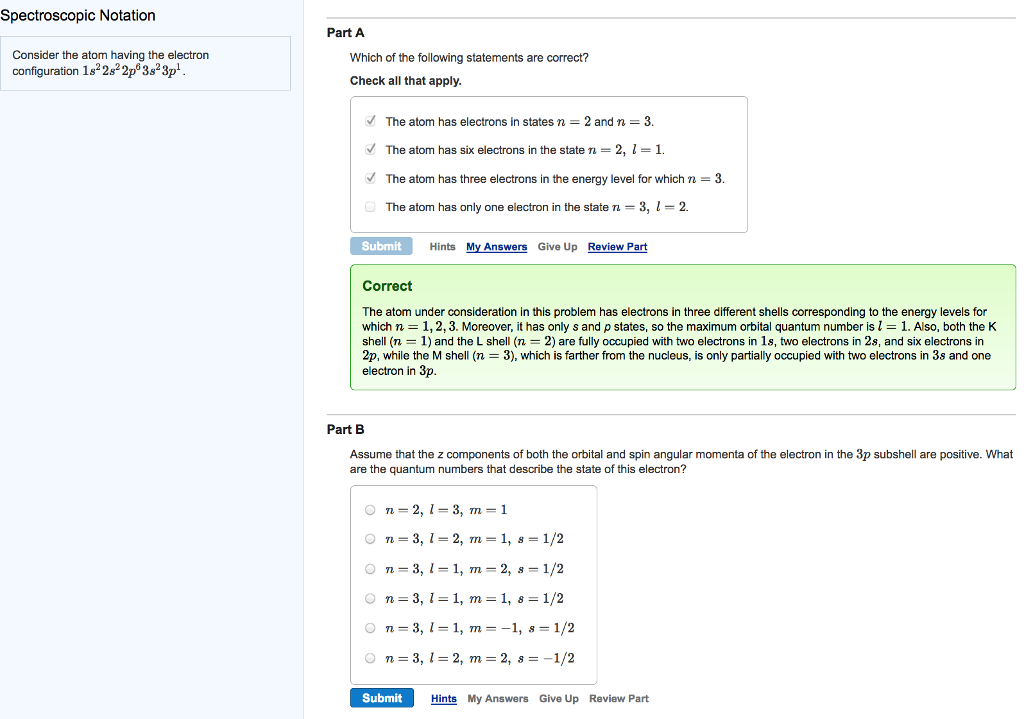
Spectroscopic Notation Consider the atom having the electron configuration 182s2p 3s3p. Part A Which of the following statements are correct? Check all that apply. The atom has electrons in states n = 2 and n = 3. The atom has six electrons in the state n = 2, l = 1. The atom has three electrons in the energy level for which n = 3. The atom has only one electron in the state n = 3, l = 2. Submit Hints My Answers Give Up Review Part Correct The atom under consideration in this problem has electrons in three different shells corresponding to the energy levels for which n = 1, 2, 3. Moreover, it has only s and p states, so the maximum orbital quantum number is 1 = 1. Also, both the K shell (n = 1) and the L shell (n = 2) are fully occupied with two electrons in 1s, two electrons in 2s, and six electrons in 2p, while the M shell (n = 3), which is farther from the nucleus, is only partially occupied with two electrons in 3s and one electron in 3p. Part B Assume that the z components of both the orbital and spin angular momenta of the electron in the 3p subshell are positive. What are the quantum numbers that describe the state of this electron? On=2, 1-3, m = 1 On 3, 1-2, m = 1, s = 1/2 On=3, 11, m2, s = 1/2 On=3, 1=1, m = 1, 8 = 1/2 On 3, 1-1, m = -1, s = 1/2 n = 3, 12, m = 2, s = -1/2 Submit Hints My Answers Give Up Review Part
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
6 15 253 38 3p The afortom Har ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


