 I need the help of an expert please!!
I need the help of an expert please!!
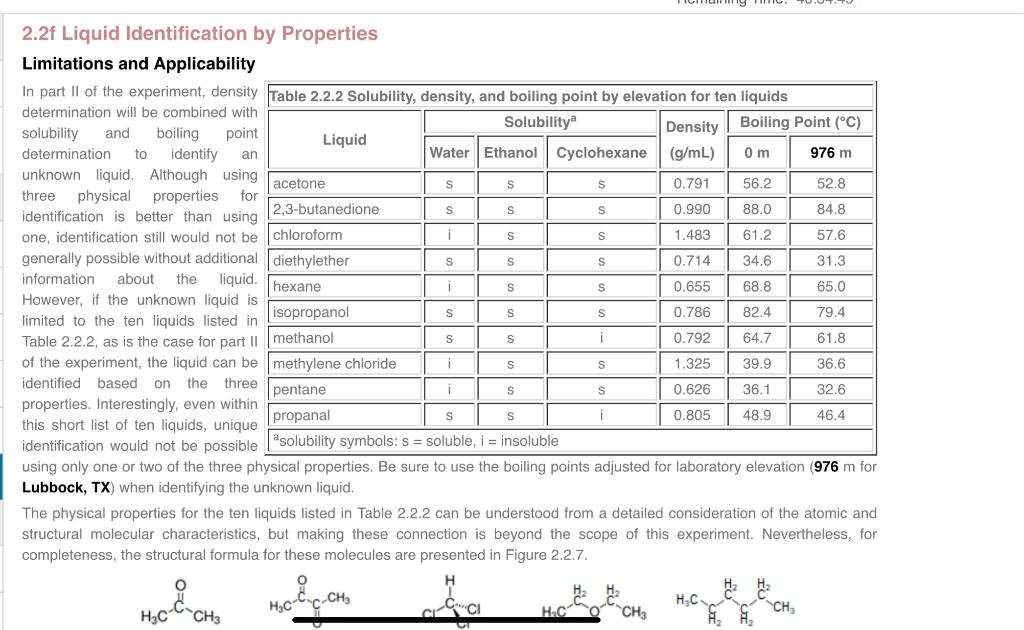
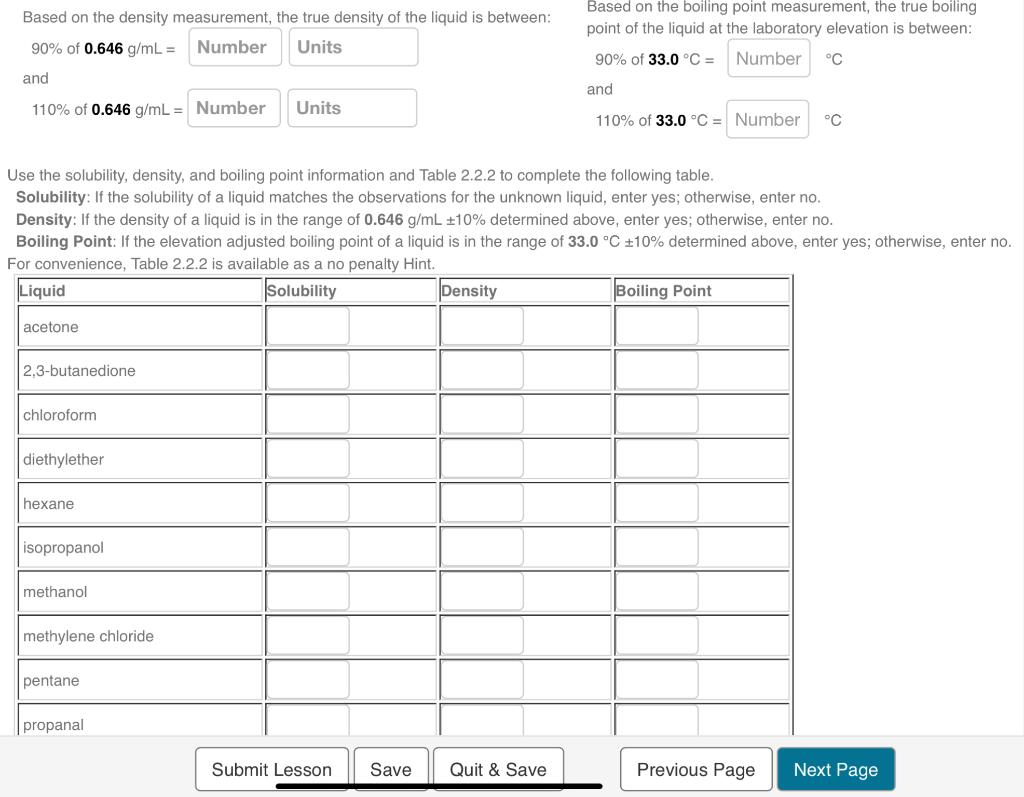
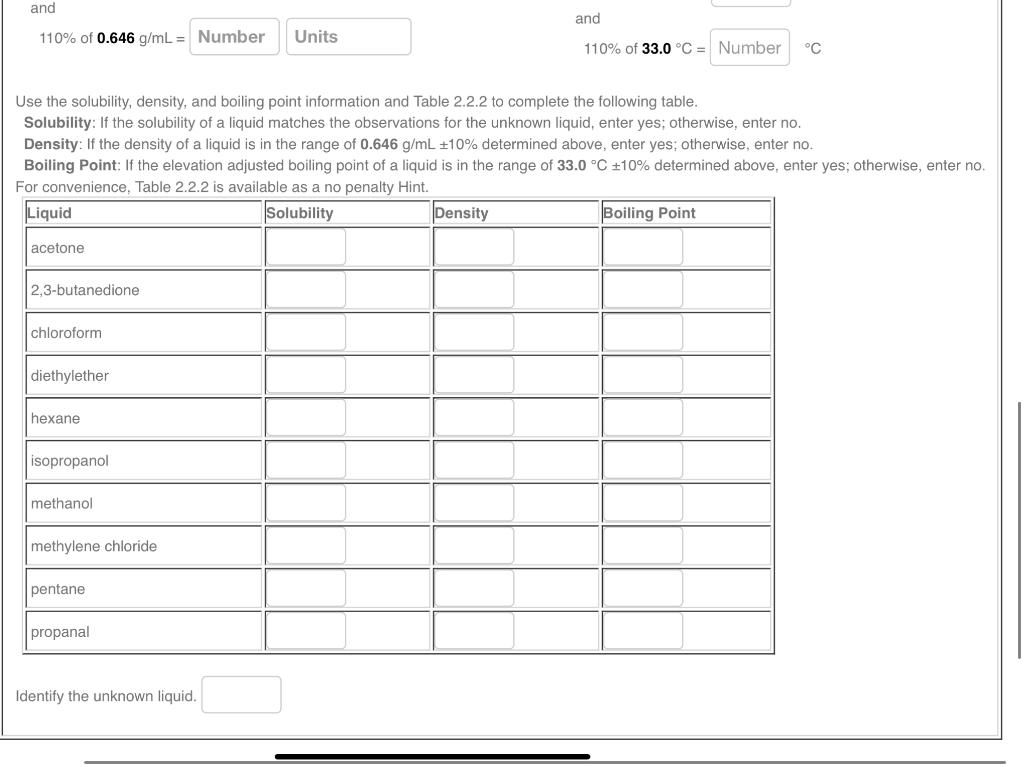
2.2f Liquid Identification by Properties Limitations and Applicability In part II of the experiment, density determination will be combined with solubility and boiling point determination to identify an unknown liquid. Although using three physical properties for identification is better than using one, identification still would not be generally possible without additional information about the liquid. However, if the unknown liquid is limited to the ten liquids listed in Table 2.2.2, as is the case for part II of the experiment, the liquid can be identified based on the three properties. Interestingly, even within this short list of ten liquids, unique identification would not be possible using only one or two of the three physical properties. Be sure to use the boiling points adjusted for laboratory elevation 976m for Lubbock, TX) when identifying the unknown liquid. The physical properties for the ten liquids listed in Table 2.2.2 can be understood from a detailed consideration of the atomic and structural molecular characteristics, but making these connection is beyond the scope of this experiment. Nevertheless, for completeness, the structural formula for these molecules are presented in Figure 2.2.7. Based on the density measurement, the true density of the liquid is between: Based on the boiling point measurement, the true boiling 90% of 0.646g/mL= point of the liquid at the laboratory elevation is between: and 110% of 0.646g/mL= 90% of 33.0C= and 110% of 33.0C= C Use the solubility, density, and boiling point information and Table 2.2.2 to complete the following table. Solubility: If the solubility of a liquid matches the observations for the unknown liquid, enter yes; otherwise, enter no. Density: If the density of a liquid is in the range of 0.646g/mL10% determined above, enter yes; otherwise, enter no. Boiling Point: If the elevation adjusted boiling point of a liquid is in the range of 33.0C10% determined above, enter yes; otherwise, enter no. For convenience. Table 2.2.2 is available as a no nenaltv Hint. Use the solubility, density, and boiling point information and Table 2.2.2 to complete the following table. Solubility: If the solubility of a liquid matches the observations for the unknown liquid, enter yes; otherwise, enter no. Density: If the density of a liquid is in the range of 0.646g/mL10% determined above, enter yes; otherwise, enter no. Boiling Point: If the elevation adjusted boiling point of a liquid is in the range of 33.0C10% determined above, enter yes; otherwise, enter no. For convenience. Table 2.2.2 is available as a no benaltv Hint. Identify the unknown liquid. 2.2f Liquid Identification by Properties Limitations and Applicability In part II of the experiment, density determination will be combined with solubility and boiling point determination to identify an unknown liquid. Although using three physical properties for identification is better than using one, identification still would not be generally possible without additional information about the liquid. However, if the unknown liquid is limited to the ten liquids listed in Table 2.2.2, as is the case for part II of the experiment, the liquid can be identified based on the three properties. Interestingly, even within this short list of ten liquids, unique identification would not be possible using only one or two of the three physical properties. Be sure to use the boiling points adjusted for laboratory elevation 976m for Lubbock, TX) when identifying the unknown liquid. The physical properties for the ten liquids listed in Table 2.2.2 can be understood from a detailed consideration of the atomic and structural molecular characteristics, but making these connection is beyond the scope of this experiment. Nevertheless, for completeness, the structural formula for these molecules are presented in Figure 2.2.7. Based on the density measurement, the true density of the liquid is between: Based on the boiling point measurement, the true boiling 90% of 0.646g/mL= point of the liquid at the laboratory elevation is between: and 110% of 0.646g/mL= 90% of 33.0C= and 110% of 33.0C= C Use the solubility, density, and boiling point information and Table 2.2.2 to complete the following table. Solubility: If the solubility of a liquid matches the observations for the unknown liquid, enter yes; otherwise, enter no. Density: If the density of a liquid is in the range of 0.646g/mL10% determined above, enter yes; otherwise, enter no. Boiling Point: If the elevation adjusted boiling point of a liquid is in the range of 33.0C10% determined above, enter yes; otherwise, enter no. For convenience. Table 2.2.2 is available as a no nenaltv Hint. Use the solubility, density, and boiling point information and Table 2.2.2 to complete the following table. Solubility: If the solubility of a liquid matches the observations for the unknown liquid, enter yes; otherwise, enter no. Density: If the density of a liquid is in the range of 0.646g/mL10% determined above, enter yes; otherwise, enter no. Boiling Point: If the elevation adjusted boiling point of a liquid is in the range of 33.0C10% determined above, enter yes; otherwise, enter no. For convenience. Table 2.2.2 is available as a no benaltv Hint. Identify the unknown liquid


 I need the help of an expert please!!
I need the help of an expert please!!





