Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need this ASAP Problem 1 (40 Pts) If we need to cure our samples at 2.5atm of CO2, what is the equilibrium pH that
I need this ASAP
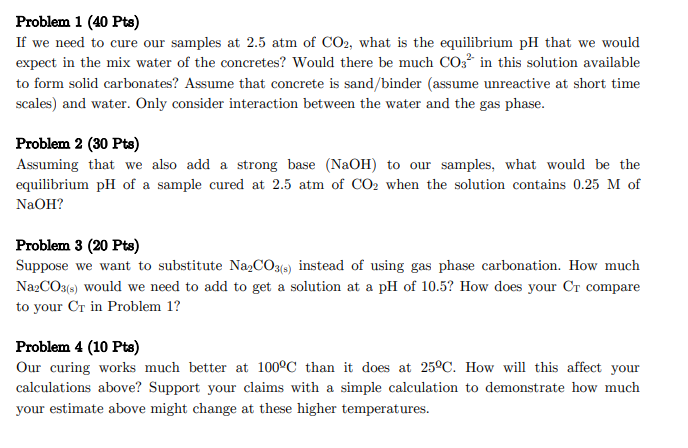
Problem 1 (40 Pts) If we need to cure our samples at 2.5atm of CO2, what is the equilibrium pH that we would expect in the mix water of the concretes? Would there be much CO32 in this solution available to form solid carbonates? Assume that concrete is sand/binder (assume unreactive at short time scales) and water. Only consider interaction between the water and the gas phase. Problem 2 (30 Pts) Assuming that we also add a strong base ( NaOH) to our samples, what would be the equilibrium pH of a sample cured at 2.5atm of CO2 when the solution contains 0.25M of NaOH? Problem 3(20Pts) Suppose we want to substitute Na2CO3(s) instead of using gas phase carbonation. How much Na2CO3(s) would we need to add to get a solution at a pH of 10.5 ? How does your CT compare to your CT in Problem 1? Problem 4 (10 Pts) Our curing works much better at 100C than it does at 25C. How will this affect your calculations above? Support your claims with a simple calculation to demonstrate how much your estimate above might change at these higher temperaturesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


