Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i post the first question but there was no clear answer so please give me the solution of both questions 1. In the reaction; (I2+2S2O32S4O62+2I),12.7g
i post the first question but there was no clear answer so please give me the solution of both questions 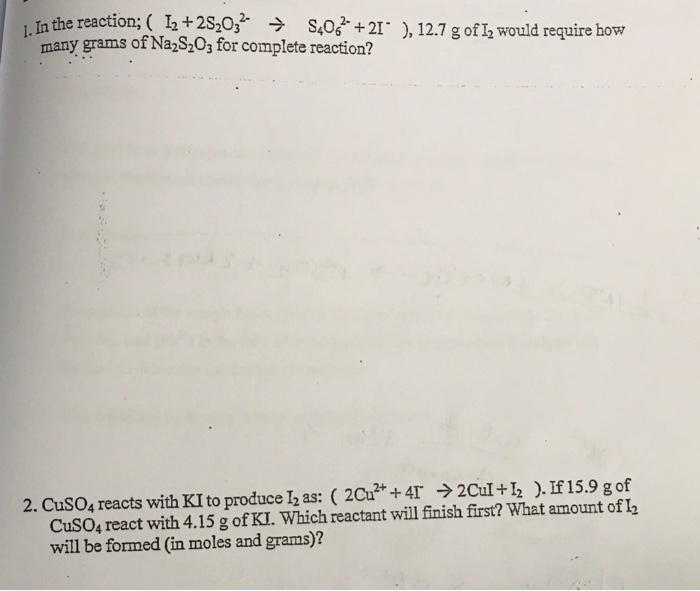
1. In the reaction; (I2+2S2O32S4O62+2I),12.7g of I2 would require how many grams of Na2S2O3 for complete reaction? 2. CuSO4 reacts with KI to produce I2 as: (2Cu2++4I2CuI+I2). If 15.9g of CuSO4 react with 4.15g of KI. Which reactant will finish first? What amount of I2 will be formed (in moles and grams) 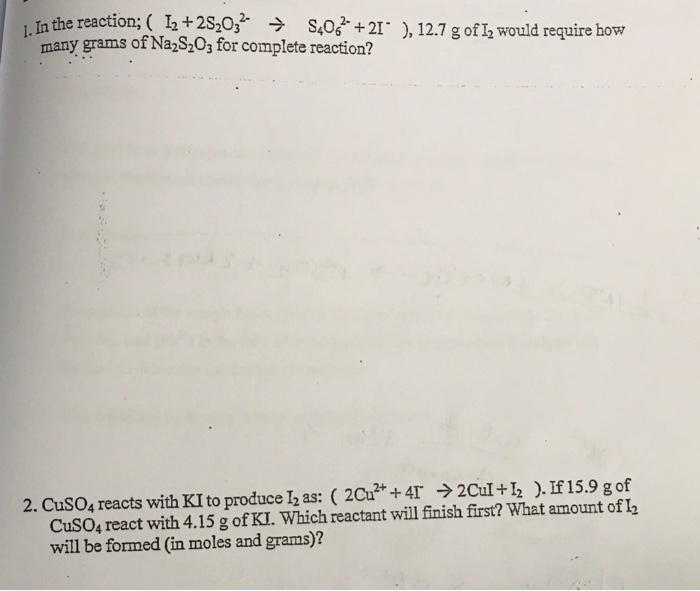
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


