Question
Consider the following reaction: HO (1) H(g) + O (g) AH = +285.8 kJ mol- Which of the following statements is correct? The reaction
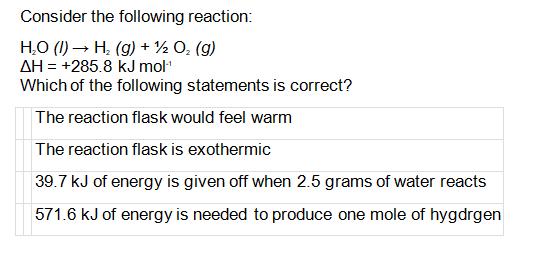
Consider the following reaction: HO (1) H(g) + O (g) AH = +285.8 kJ mol- Which of the following statements is correct? The reaction flask would feel warm The reaction flask is exothermic 39.7 kJ of energy is given off when 2.5 grams of water reacts 571.6 kJ of energy is needed to produce one mole of hygdrgen
Step by Step Solution
3.33 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The reaction is endothermic because the enthalpy change is positive ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Ethics in Accounting A Decision Making Approach
Authors: Gordon Klein
1st edition
1118928334, 978-1118928332
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



