I would appreciate if someone could check my work for finding the reactant molarities, rate of reaction, and what is the rate constant on photo #3. Thank you!!
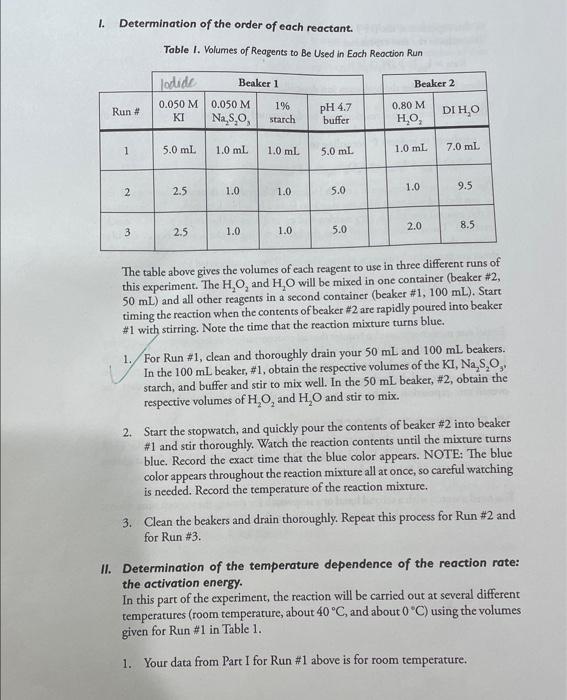

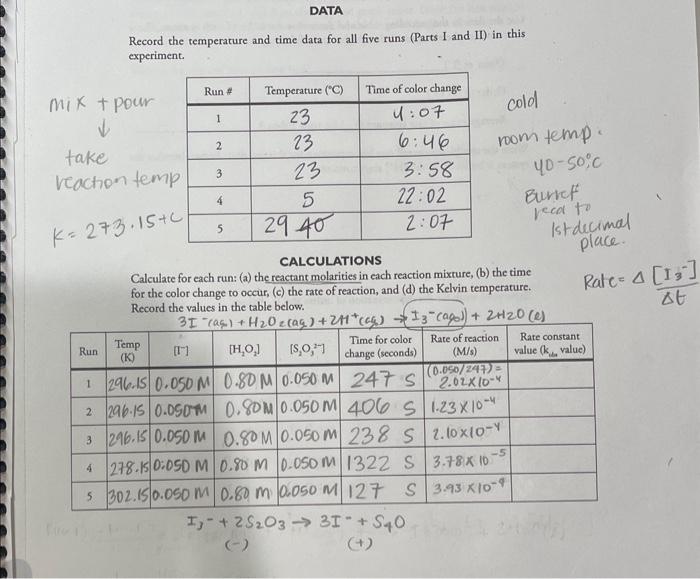


I. Determination of the order of each reactant. Table I. Volumes of Reagents to Be Used in Each Reaction Run The table above gives the volumes of each reagent to use in three different runs of this experiment. The H2O2 and H2O will be mixed in one container (beaker \#2, 50mL ) and all other reagents in a second container (beaker #1,100mL ). Start timing the reaction when the contents of beaker #2 are rapidly poured into beaker \#1 with stirring. Note the time that the reaction mixture turns blue. 1. For Run \#1, clean and thoroughly drain your 50mL and 100mL beakers. In the 100mL beaker, \#1, obtain the respective volumes of the KI,Na2S2O3, starch, and buffer and stir to mix well. In the 50mL beaker, #2, obtain the respective volumes of H2O2 and H2O and stir to mix. 2. Start the stopwatch, and quickly pour the contents of beaker \#2 into beaker \#1 and stir thoroughly. Watch the reaction contents until the mixture turns blue. Record the exact time that the blue color appears. NOTE: The blue color appears throughout the reaction mixture all at once, so careful watching is needed. Record the temperature of the reaction mixture. 3. Clean the beakers and drain thoroughly. Repeat this process for Run \#2 and for Run 3 . II. Determination of the temperature dependence of the reaction rate: the activation energy. In this part of the experiment, the reaction will be carried out at several different temperatures (room temperature, about 40C, and about 0C ) using the volumes given for Run \# 1 in Table 1. 2. Obtain the volumes of reagents needed for Run \#1 in two small beakers (or small Erlenmeyer flasks). Set the beakers in a tray of ice water (about one inch deep) for 3-5 minutes, stirring occasionally. Allow the temperature of the solutions in the beakers to stabilize and record this temperature. Pour the contents of beaker #2 into beaker \#1, stir well and immediately start the stopwatch. Leave the reaction mixture in the tray of ice water and stir occasionally. Record the time at which the reaction mixture turns blue. Note: Reactions occur slower at lower temperatures so this will take longer. Clean and drain your beakers. PUT ALL WASTE SOLUTIONS IN THE APPROPRIATE WASTE CONTAINER. 3. Obtain the volumes of reagents needed for Run \#1 in two small beakers. Prepare a "hot water bath" by heating 300mL of water in a 600mL beaker to near boiling using a Bunsen burner. Then use a folded paper towel to transfer the beaker and pour the hot water into one of the water-bath trays. Then add enough room temperature water to bring the bath temperature to about 40C. Set beakers #1 and #2 in the hot water bath for 35 minutes, stir occasionally, allow the temperature to stabilize and record this temperature. Pour the contents of beaker #2 into beaker #1, stir well and immediately start the stopwatch. Leave the reaction mixture in the hot water bath and stir occasionally. Record the time the reaction mixture turns blue. Note: Reactions occur more rapidly at higher temperatures, so watch this closely. Clean and drain your beakers. II.DATA WRITE-UP Part I. Use the method of initial rates and determine the complete rate law expression for this reaction. Part II. Review Laboratory Techniques: Graphing in your lab manual. Make a graph of ln k vs. 1/T for runs 1,4 and 5 using the supplied graph paper. Draw the best straight line. On the graph, show how you determined the slope of the line. Also show how you calculated the value of the activation energy from the slope of the graph. Record the temperature and time data for all five runs (Parts I and II) in this experiment. pour cold room temp. 4050C Burbef recal to |st decimal place. CALCULATIONS Calculate for each run: (a) the reactant molarities in each reaction mixture, (b) the time for the color change to occur, (c) the rate of reaction, and (d) the Kelvin temperature. I. Determination of the rate law expression. Write the balanced chemical equation for the reaction being studied in this experiment. 3I(aq)+H2O2(ag8)+2H+(ag0)I3(ag)+2H2O(e) Use the data in the preceding table and determine the order of each reactant. Show your work in the area provided at the bottom of the page. Order of [I] Order of [H2O2] Average value of rate constant (kobb) for runs 1 thru 3 Write the completed overall rate law expression for this reaction. Remember that the system is buffered, so the [H+]stays constant. Show your work for the determination of orders and kob values: II. Determination of the activation energy. Review Laboratory Techniques: Graphing in your lab manual. Prepare a graph of lnkobs versus 1/T for runs 1,4 , and 5 on the supplied graph paper. Draw the best straight line. On the graph, show how you determined the slope of the line. Also show how you calculated the value of the activation energy from the slope of the graph. What is the slope of your plotted line? What is your calculated Ea value











