Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, and the universal gas constant R = 0.0821 Latm to solve the
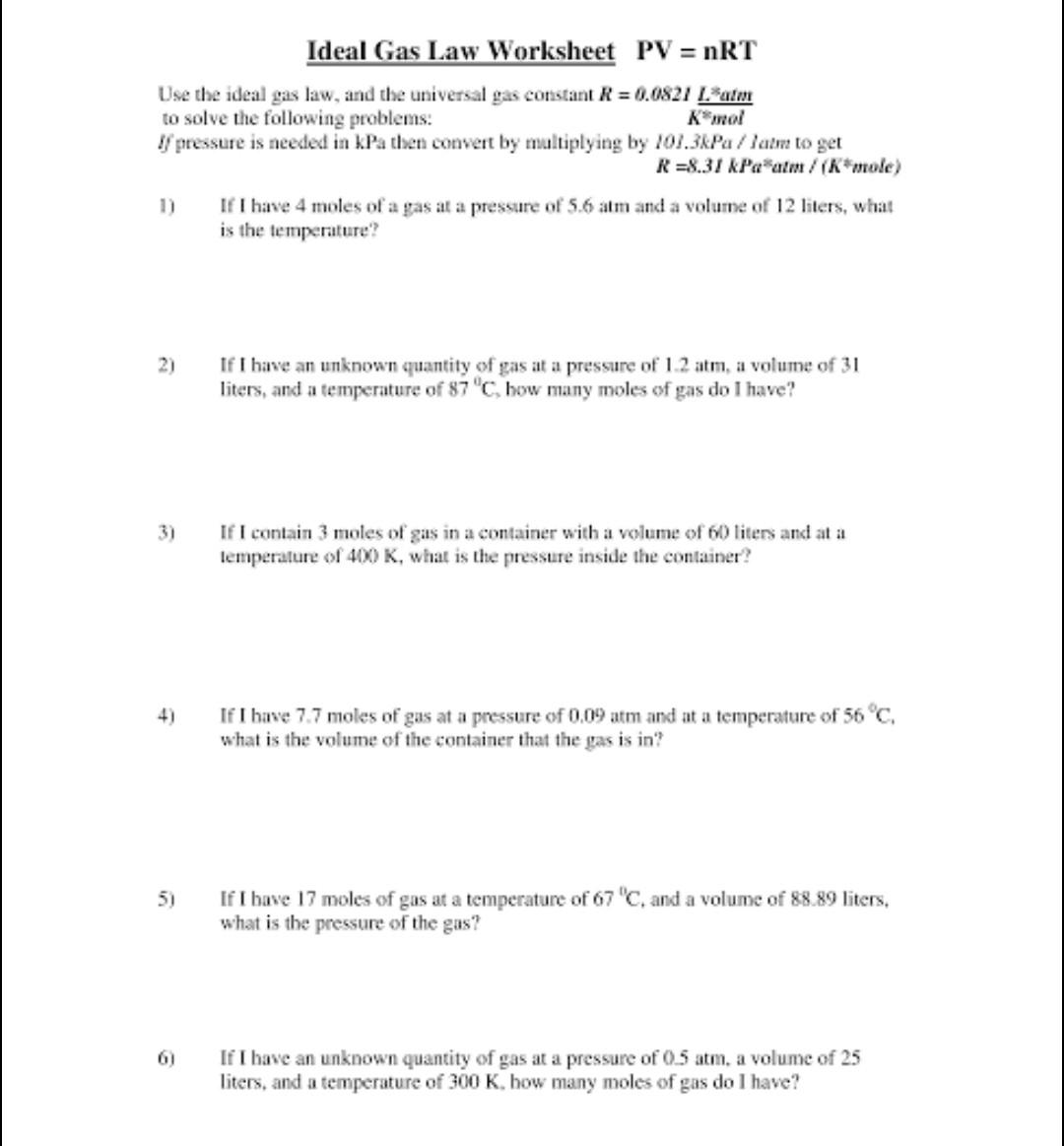
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, and the universal gas constant R = 0.0821 Latm to solve the following problems: K mol If pressure is needed in kPa then convert by multiplying by 101.3kPa/latm to get R=8.31 kPa atm / (K*mole) 2) 3) 5) 6) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters, what is the temperature? If I have an unknown quantity of gas at a pressure of 1.2 atm, a volume of 31 liters, and a temperature of 87 "C, how many moles of gas do I have? If I contain 3 moles of gas in a container with a volume of 60 liters and at a temperature of 400 K, what is the pressure inside the container? If I have 7.7 moles of gas at a pressure of 0.09 atm and at a temperature of 56 C, what is the volume of the container that the gas is in? If I have 17 moles of gas at a temperature of 67 "C, and a volume of 88.89 liters, what is the pressure of the gas? If I have an unknown quantity of gas at a pressure of 0.5 atm, a volume of 25 liters, and a temperature of 300 K, how many moles of gas do I have?
Step by Step Solution
★★★★★
3.43 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
Sure Lets solve each problem step by step using the ideal gas law equation PV nRT Remember to use the appropriate units and convert them if necessary ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


