Question
Consider the following data on some weak acids and weak bases: formula hypochlorous acid HCIO hydrofluoric acid. HF 0.1 M KCI solution 0.1 M
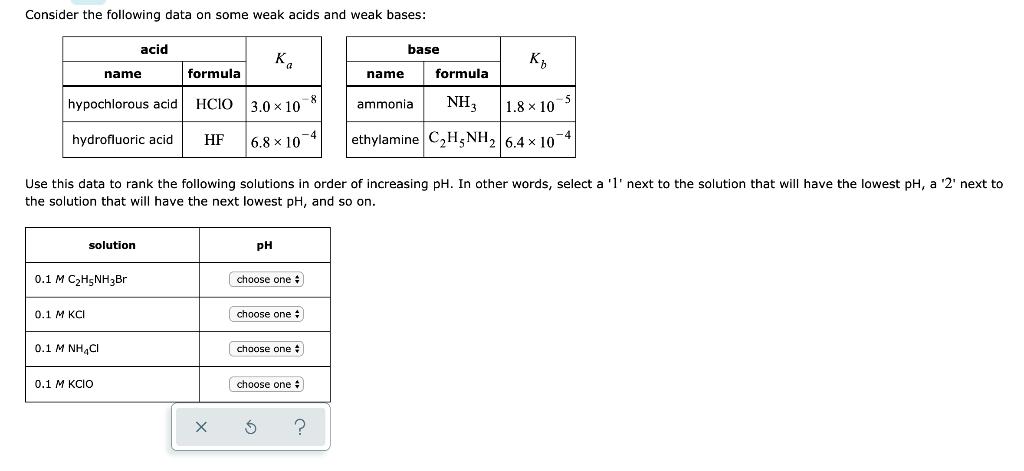
Consider the following data on some weak acids and weak bases: formula hypochlorous acid HCIO hydrofluoric acid. HF 0.1 M KCI solution 0.1 M CH5NHBr acid name 0.1 MINH,C 0.1 M KCIO X Ka 3.0 x 10 6.8x10 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. PH choose one # choose one choose one choose one # $ ? base K NH3 1.8 10 ethylamine CH5NH 6.4 x 10 name ammonia formula -5
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
pH of 01 M NaCl NaCl is a salt 9f strong acid and strong base The salt solution is neut...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Exploring Economics
Authors: Robert L Sexton
5th Edition
978-1439040249, 1439040249
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



