Question
Identify the conjugate base of formic acid (HCOOH). HCOO HCOO COOH HCOOH, Complete the balanced equation for the reaction that occurs when formic acid
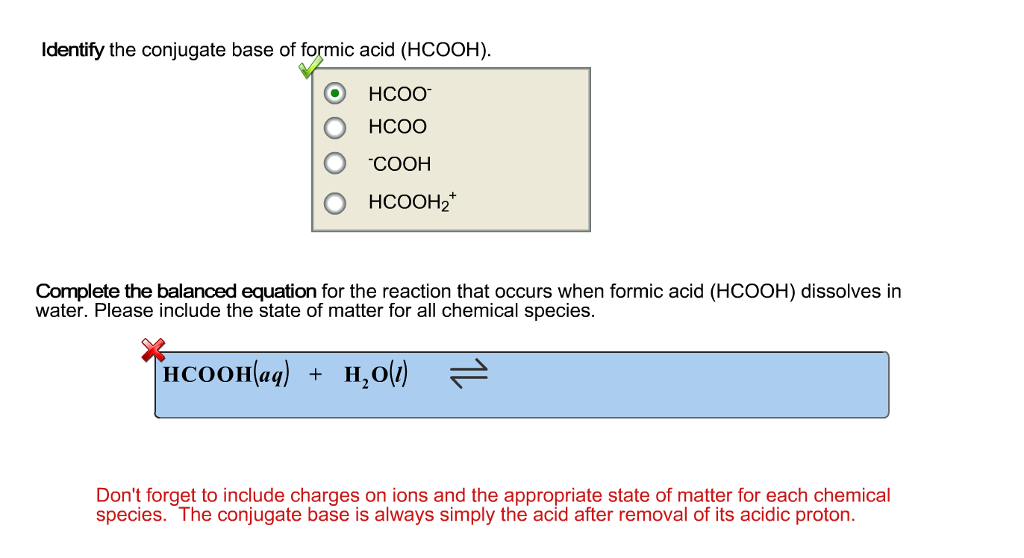
Identify the conjugate base of formic acid (HCOOH). HCOO HCOO COOH HCOOH, Complete the balanced equation for the reaction that occurs when formic acid (HCOOH) dissolves in water. Please include the state of matter for all chemical species. HCOOHaq) + HO(1) Don't forget to include charges on ions and the appropriate state of matter for each chemical species. The conjugate base is always simply the acid after removal of its acidic proton.
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
HCOOH PAGE DATE ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Marc Loudon
5th edition
981519431, 978-0981519449, 098151944X, 978-0-98151943, 978-0981519432
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



