Question
Identify the effect of increasing acidity on the solubility of the given compounds. Drag the appropriate items to their respective bins. MgBr2 Cul Ca3(PO4)2
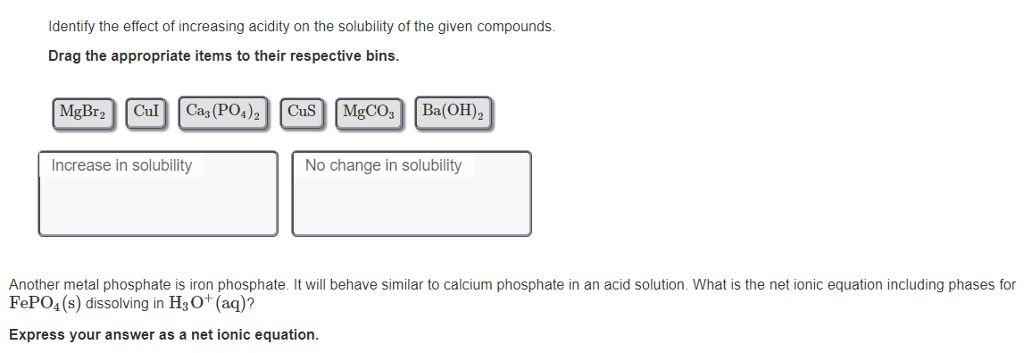
Identify the effect of increasing acidity on the solubility of the given compounds. Drag the appropriate items to their respective bins. MgBr2 Cul Ca3(PO4)2 CuS MgCO3 Increase in solubility Ba(OH)2 No change in solubility Another metal phosphate is iron phosphate. It will behave similar to calcium phosphate in an acid solution. What is the net ionic equation including phases for FePO4(s) dissolving in H3O+ (aq)? Express your answer as a net ionic equation.
Step by Step Solution
3.52 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
Net Ionic Equation FePO 4 s H 3 O aq Fe 3 aq HPO 4 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting Introduction To Financial Accounting
Authors: Henry Dauderis, David Annand
1st Edition
1517089719, 978-1517089719
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



