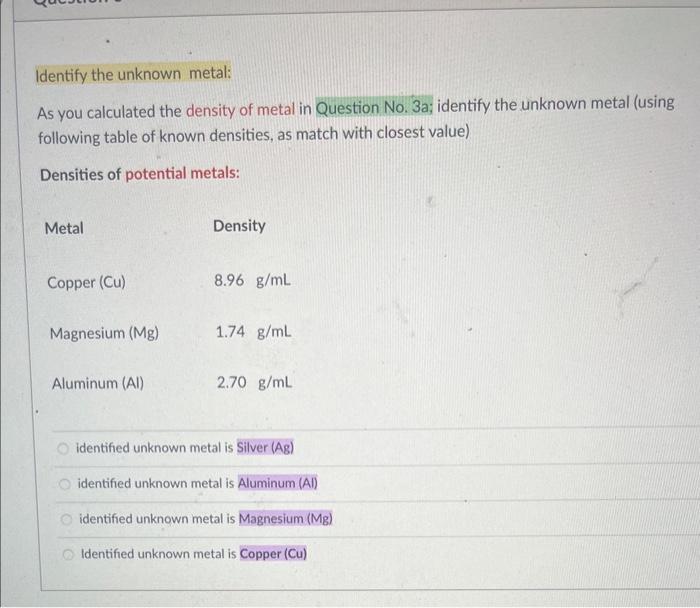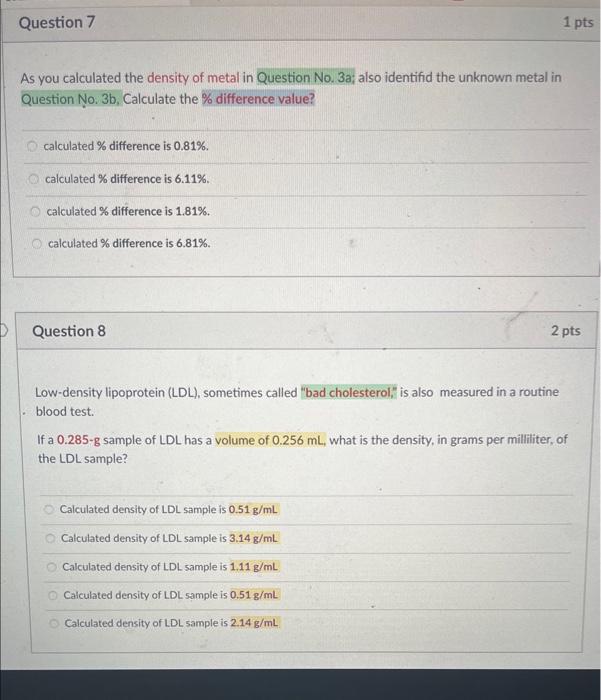
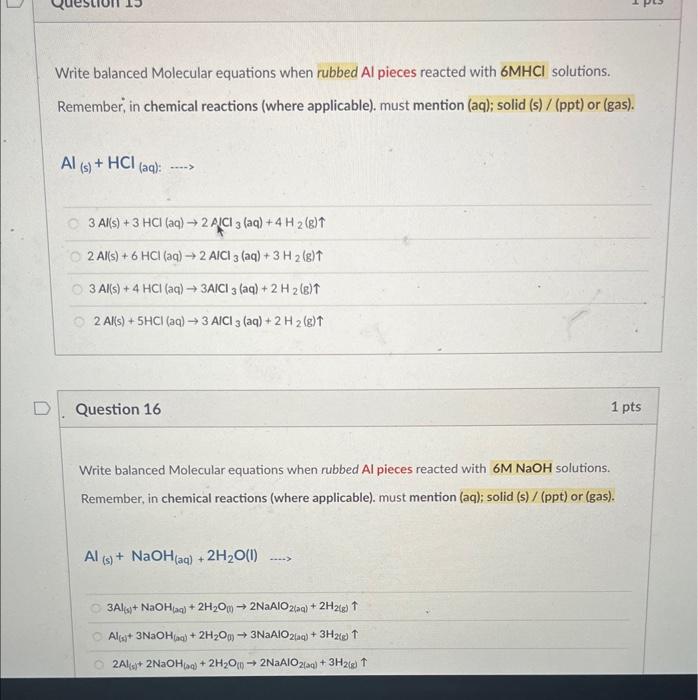
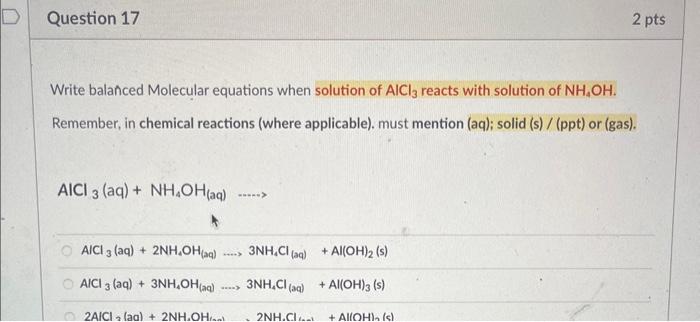
Identify the unknown metal: As you calculated the density of metal in Question No. 3a; identify the unknown metal (using following table of known densities, as match with closest value) Densities of potential metals: identified unknown metal is Silver (Ag) identified unknown metal is Aluminum (A) identified unknown metal is Magnesium ( Mg ) Identified unknown metal is Copper (Cu) As you calculated the density of metal in Question No. 3a; also identifid the unknown metal in Question No. 3b, Calculate the % difference value? calculated % difference is 0.81%. calculated \% difference is 6.11%. calculated \% difference is 1.81%. calculated \% difference is 6.81%. Question 8 2 pts Low-density lipoprotein (LDL), sometimes called "bad cholesterol," is also measured in a routine - blood test. If a 0.285g sample of LDL has a volume of 0.256mL, what is the density, in grams per milliliter, of the LDL sample? Calculated density of LDL sample is 0.51g/mL Calculated density of LDL sample is 3.14g/mL Calculated density of LDL sample is 1.11g/mL Calculated density of LDL sample is 0.51g/mL Calculated density of LDL sample is 2.14g/mL Write balanced Molecular equations when rubbed Al pieces reacted with 6MHCl solutions. Remember, in chemical reactions (where applicable). must mention (aq); solid (s) / (ppt) or (gas). Al(s)+HCl(aq):...) 3Al(s)+3HCl(aq)2ACl3(aq)+4H2(g)2Al(s)+6HCl(aq)2AICl3(aq)+3H2(g)3Al(s)+4HCl(aq)3AICl3(aq)+2H2(g)2Al(s)+5HCl(aq)3AlCl3(aq)+2H2(g) Question 16 1 pts Write balanced Molecular equations when rubbed Al pieces reacted with 6MNaOH solutions. Remember, in chemical reactions (where applicable). must mention (aq); solid (s) / (ppt) or (gas). Al(s)+NaOH(aq)+2H2O(l).3Al(s)+NaOH(aq)+2H2O(0)2NaAlO2(a)+2H2(8)Al(s)+3NaOH(a)+2H2Op)3NaAlO2(aq)+3H2(s)2Al(s)+2NaOH(a)+2H2O(n)2NaAlO2(sa)+3H2(s) Write balanced Molecular equations when solution of AlCl3 reacts with solution of NH4OH. Remember, in chemical reactions (where applicable). must mention (aq); solid (s) / (ppt) or (gas). AlCl3(aq)+NH4OH(aq)... AlCl3(aq)+2NH4OH(aq)3NH4Cl(aq)+Al(OH)2(s)AlCl3(aq)+3NH4OH(aq)3NH4Cl(aq)+Al(OH)3(s)