Answered step by step
Verified Expert Solution
Question
1 Approved Answer
idk if you need this table to solve for the theoretical value. how do you do the experimental part and the theoretical part? Part A
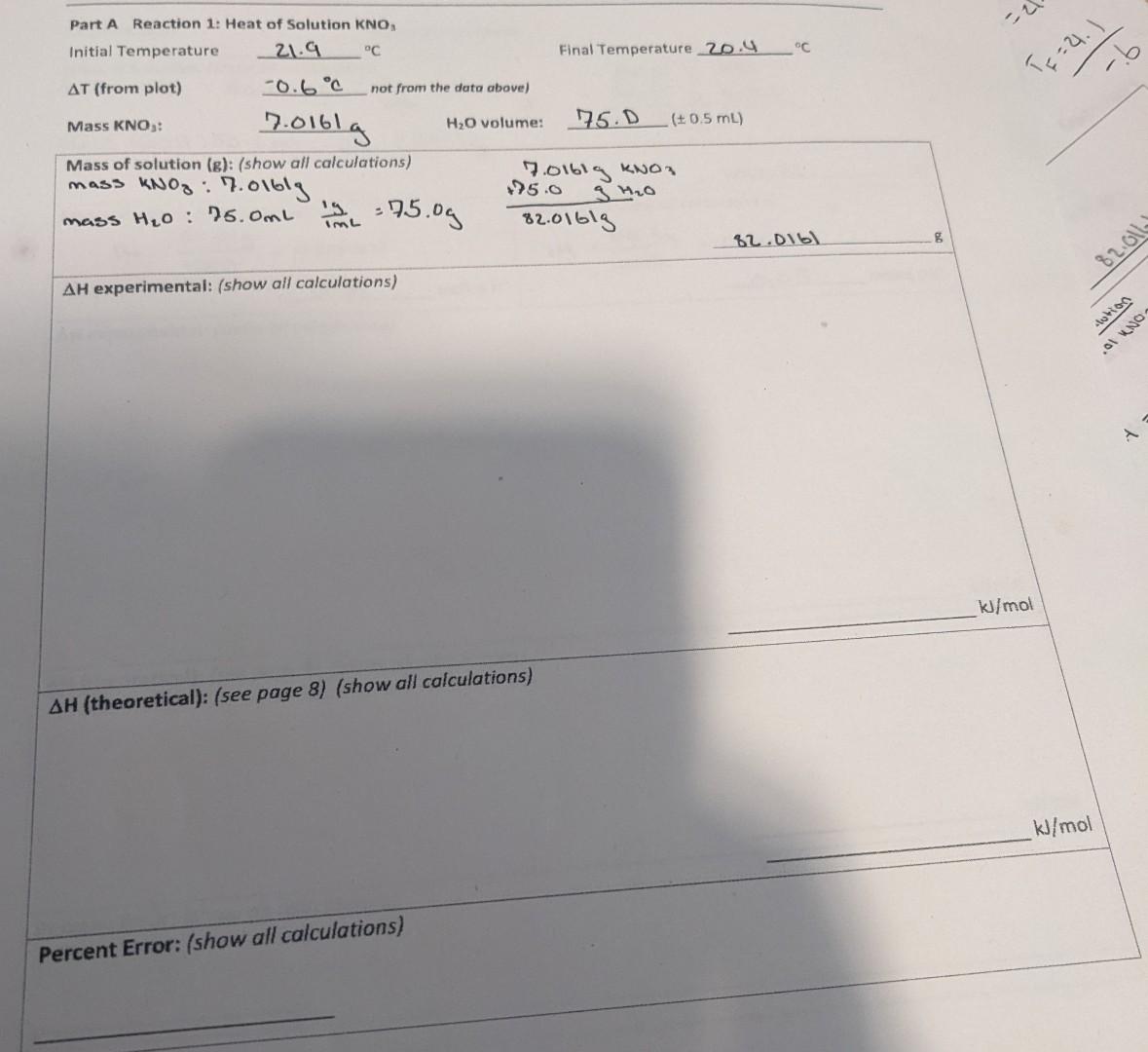
idk if you need this table to solve for the theoretical value.
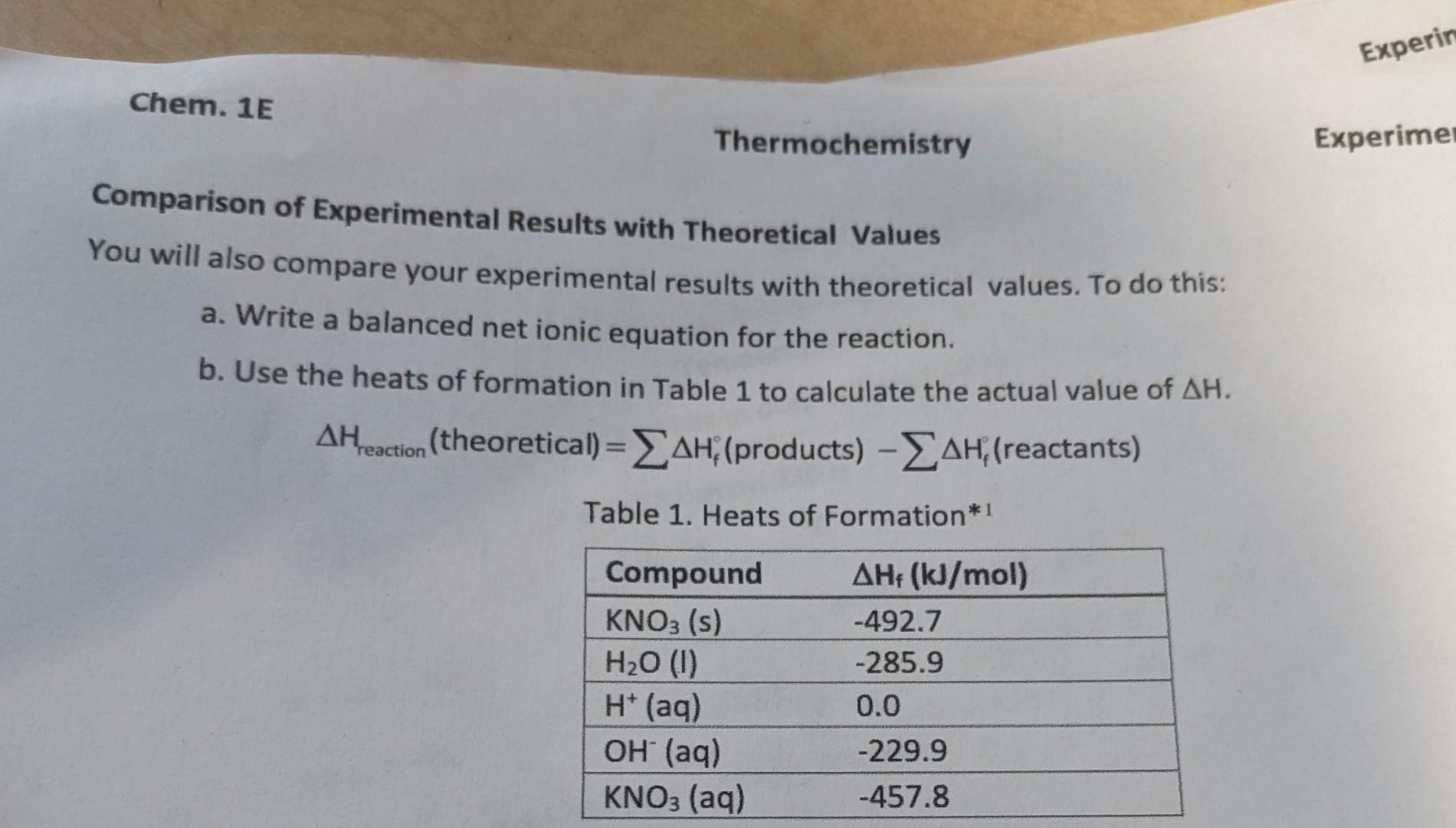
how do you do the experimental part and the theoretical part?
Part A Reaction 1: Heat of Solution KNO, H experimental: (show all calculations) H (theoretical): (see page 8) (show all calculations) Comparison of Experimental Results with Theoretical Values You will also compare your experimental results with theoretical values. To do this: a. Write a balanced net ionic equation for the reaction. b. Use the heats of formation in Table 1 to calculate the actual value of H. Hreaction(theoretical)=Hf(products)Hf(reactants) Table 1. Heats of Formation*1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


