Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If 1 mole of gas is feed to the rector, calculate the total moles in the reactor after equilibrium is reached. Calculate the total moles
If 1 mole of gas is feed to the rector, calculate the total moles in the reactor after equilibrium is reached.
Calculate the total moles of gas in the reactor at equilibrium
then the equilibrium mole fraction of hydrogen in the product.
PLEase be very very detailed on the calculations ! thanks
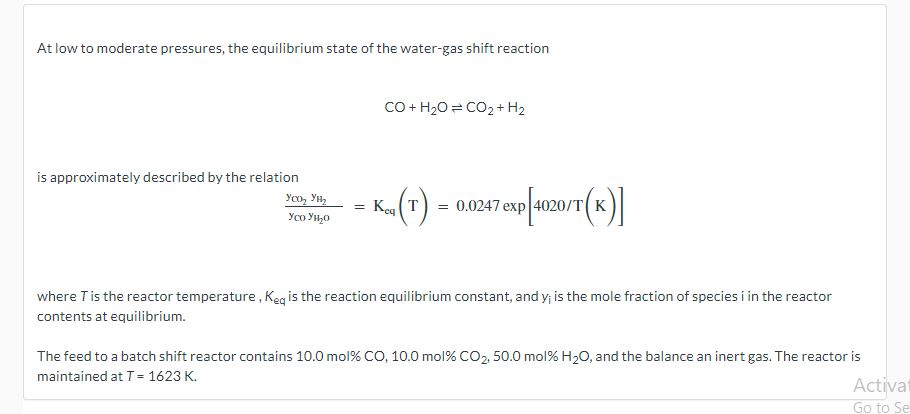
At low to moderate pressures, the equilibrium state of the water-gas shift reaction CO+H2OCO2+H2 is approximately described by the relation yCOyH2OyCO2yH2=Keq(T)=0.0247exp[4020/T(K)] where T is the reactor temperature, Keq is the reaction equilibrium constant, and yi is the mole fraction of species i in the reactor contents at equilibrium. The feed to a batch shift reactor contains 10.0mol%CO,10.0mol%CO2,50.0mol%H2O, and the balance an inert gas. The reactor is maintained at T=1623K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


