Question
If 23.2 ml of NaOH solution is required to neutralize 0.123 g of KHP (molar mass 204.2 g/mol), then the molarity of NaOH is:

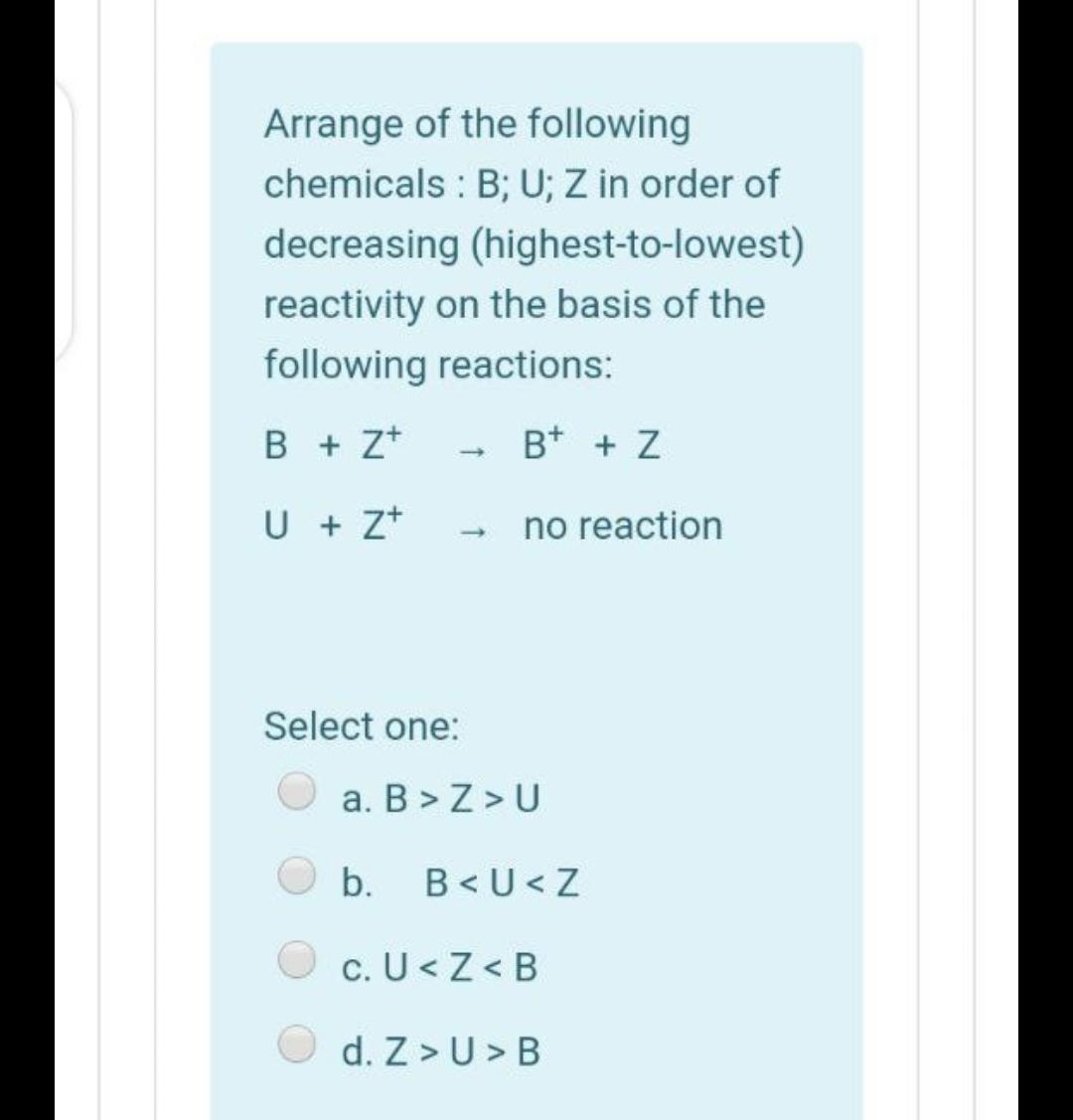
If 23.2 ml of NaOH solution is required to neutralize 0.123 g of KHP (molar mass 204.2 g/mol), then the molarity of NaOH is: Select one: a. 0.026 b. 2.60 c. 0.922 d. 0.014 Arrange of the following chemicals : B; U; Z in order of decreasing (highest-to-lowest) reactivity on the basis of the following reactions: B + z* B+ + Z U + z+ no reaction Select one: a. B > Z > U b. B B
Step by Step Solution
3.45 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



