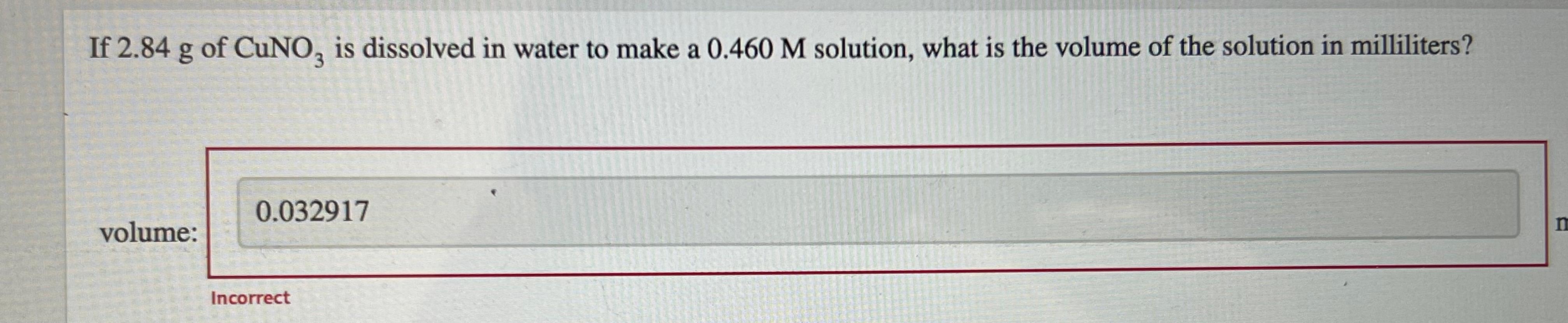




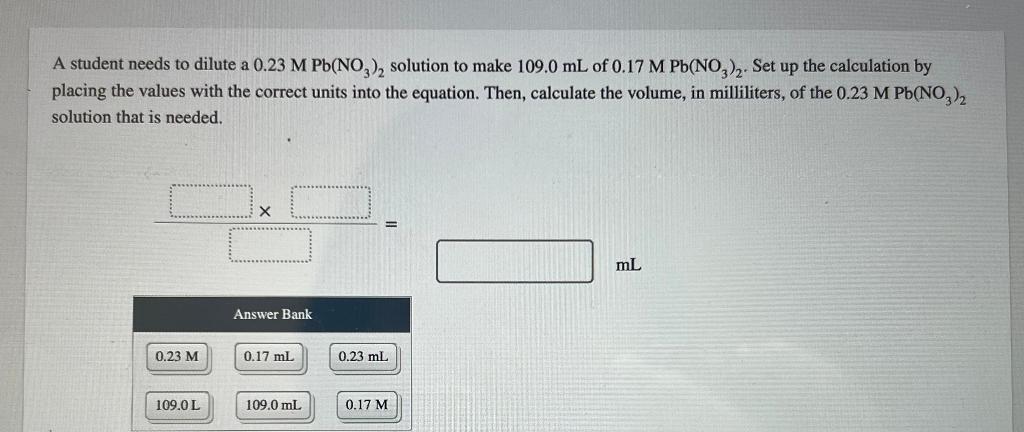






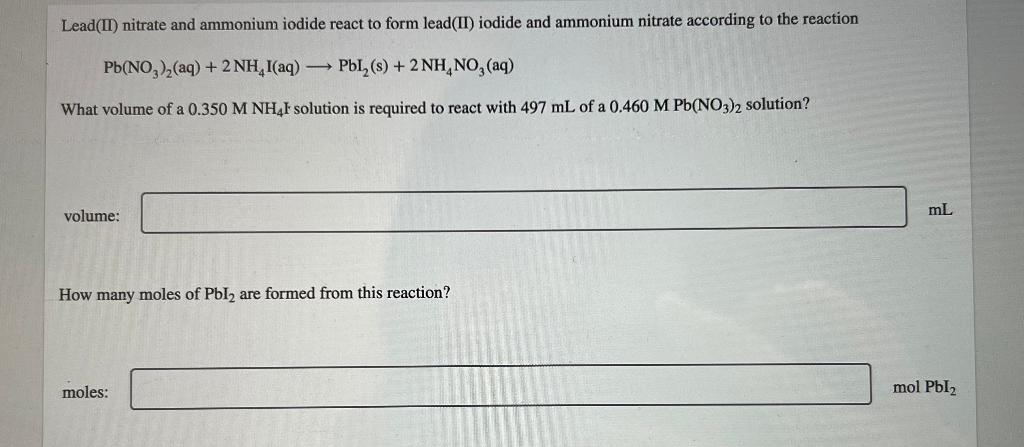
If 2.84 g of CuNoz is dissolved in water to make a 0.460 M solution, what is the volume of the solution in milliliters? a 3 0.032917 volume: Incorrect If 3.01 mL of 10.0 M NaOH is used for a reaction, calculate the number of moles of NaOH that were used. a mol number of moles of NaOH = How many grams of sodium hydroxide are present in 250.0 mL of a 0.500 M NaOH solution? mass of sodium hydroxide = g The equation for dilution of a solution is MV = M2V2 where M, and V are the initial concentration and volume, respectively, and M2 and V2 represent the final concentration and volume, respectively. When preparing a dilute solution, it is necessary to first calculate the volume of stock solution needed. Which of the expressions is equal to the volume of stock solution, V/? MV M M2V2 M M M2 V The dilution equation, rearranged to solve for Vis Vi = M2V2 M where M and V are the initial concentration and volume, respectively, and M, and V, represent the final concentration and volume, respectively. A student needs to prepare 50.0 mL of 0.80 M aqueous H,O, solution from a 5.10 MH,O, stock solution. Identify the values of M2, V2, and M in this situation. M M2 = mL V = M M A student needs to dilute a 0.23 M Pb(NO3), solution to make 109.0 mL of 0.17 M Pb(NO3)2. Set up the calculation by placing the values with the correct units into the equation. Then, calculate the volume, in milliliters, of the 0.23 M Pb(NO3)2 solution that is needed. mL Answer Bank 0.23 M 0.17 ml 0.23 ml 109.0L 109.0 mL 0.17 M What is the concentration of a solution made by diluting 25 mL of 6.0 M HCl to a final volume of 750 mL? concentration: M A student needs to prepare 50.0 mL of a 1.10 M aqueous H,Osolution. Calculate the volume of 5.1 MH, O, stock solution that should be used to prepare the solution. volume: mL Complete the equation for the dissociation of K PO, (aq). Omit water from the equation because it is understood to be present. equation: K,PO4 (aq) Calculate the lithium ion (Lit) concentration for a 0.318 M Li S solution. M [Li+] = K, SO, is a strong electrolyte. Determine the concentration of each of the individual ions in a 0.350 M K,SO, solution. [K] = M [S02 ) = M Strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride, according to the reaction shown. SrCl, (aq) + 2 NaF(aq) SrF,(s) + 2 NaCl(aq) What volume of a 0.630 M NaF solution is required to react completely with 383 mL of a 0.160 M SCL solution? ml volume: How many moles of SrF, are formed from this reaction? mol moles of SrF, Lead(II) nitrate and ammonium iodide react to form lead(II) iodide and ammonium nitrate according to the reaction Pb(NO )2(aq) + 2NH,I(aq) Pl_(s) + 2NH, NO, (aq) What volume of a 0.350 M NH I solution is required to react with 497 mL of a 0.460 M Pb(NO3)2 solution? volume: mL How many moles of Pbly are formed from this reaction? moles: mol Pbl2



















