Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If a face-centered cubic crystal structure has all of its octahedral sites filled with atoms that fit perfectly into each octahedral site, what is the
If a face-centered cubic crystal structure has all of its octahedral sites filled with atoms that fit perfectly into each octahedral site, what is the atomic packing factor?
APF = (number of atoms per unit cell x atomic volume) / unit cell volume
There are four atoms per unit cell in the FCC structure with a radius R. These are four octahedral sites in the FCC structure with radius r. The unit cell has a volume 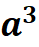 (a is the lattice parameter or axial length of the unit cell). For the FCC structure,
(a is the lattice parameter or axial length of the unit cell). For the FCC structure, 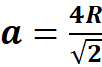
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


