Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If a substance, at 1.0 atm of pressure is cooled from 400 K to 200K, (see the phase diagram below) what phase change will
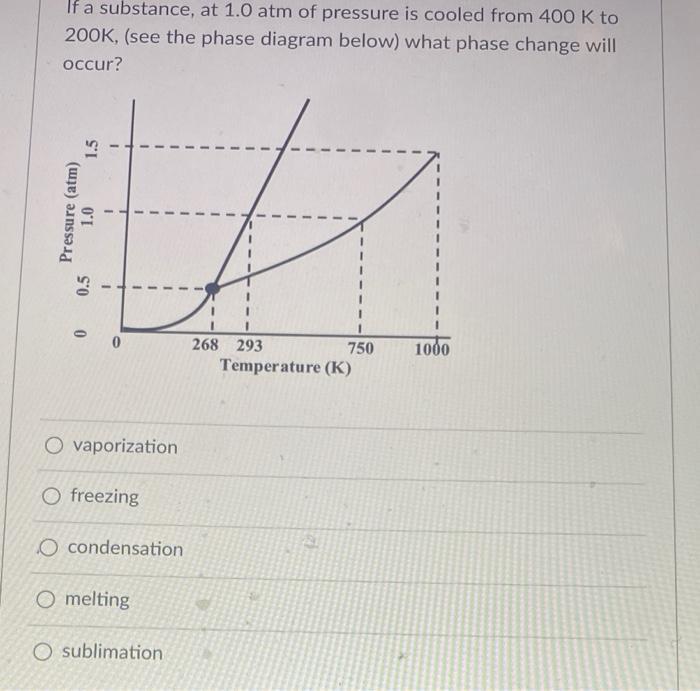
If a substance, at 1.0 atm of pressure is cooled from 400 K to 200K, (see the phase diagram below) what phase change will occur? %3D 268 293 750 1000 Temperature (K) O vaporization O freezing O condensation O melting O sublimation Pressure (atm) 0.5 1.0 1.5
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636501d8509d5_239833.pdf
180 KBs PDF File
636501d8509d5_239833.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


