Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If possible, could you explain in detail each answer? If not that's okay. Thank you! The table above contains the HfGf for several nitrogen oxides.
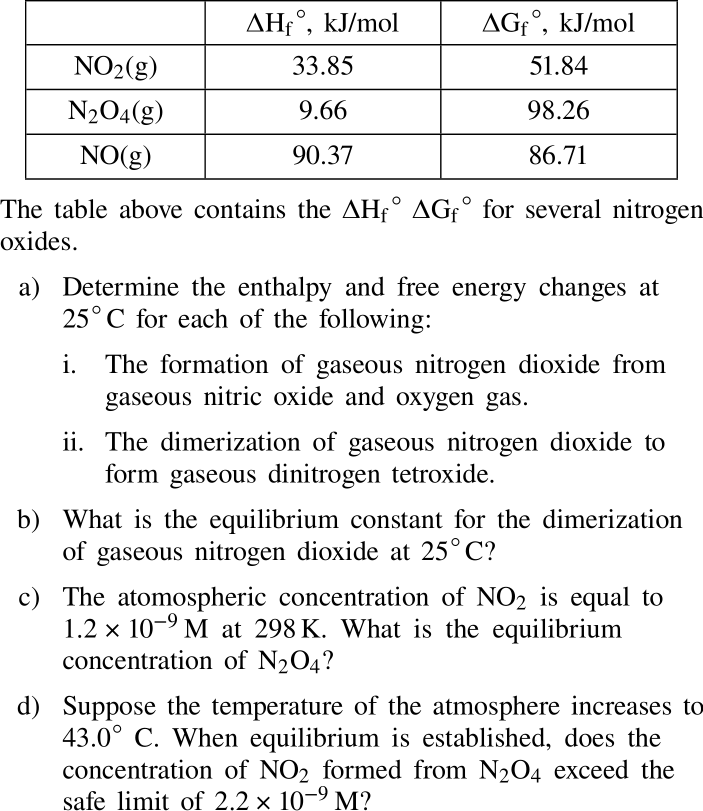
If possible, could you explain in detail each answer? If not that's okay. Thank you!
The table above contains the HfGf for several nitrogen oxides. a) Determine the enthalpy and free energy changes at 25C for each of the following: i. The formation of gaseous nitrogen dioxide from gaseous nitric oxide and oxygen gas. ii. The dimerization of gaseous nitrogen dioxide to form gaseous dinitrogen tetroxide. b) What is the equilibrium constant for the dimerization of gaseous nitrogen dioxide at 25C ? c) The atomospheric concentration of NO2 is equal to 1.2109M at 298K. What is the equilibrium concentration of N2O4 ? d) Suppose the temperature of the atmosphere increases to 43.0C. When equilibrium is established, does the concentration of NO2 formed from N2O4 exceed the safe limit of 2.2109M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


