Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Partial pressures: When we have a mixture of gases, each gas exerts a pressure according to its own number of molecules, temperature and volume.The
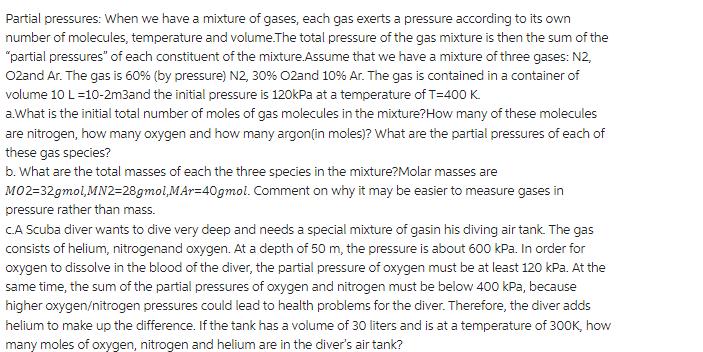
Partial pressures: When we have a mixture of gases, each gas exerts a pressure according to its own number of molecules, temperature and volume.The total pressure of the gas mixture is then the sum of the "partial pressures" of each constituent of the mixture.Assume that we have a mixture of three gases: N2, 02and Ar. The gas is 60% (by pressure) N2, 30% O2and 10% Ar. The gas is contained in a container of volume 10 L=10-2m3and the initial pressure is 120kPa at a temperature of T=400 K a.What is the initial total number of moles of gas molecules in the mixture? How many of these molecules are nitrogen, how many oxygen and how many argon (in moles)? What are the partial pressures of each of these gas species? b. What are the total masses of each the three species in the mixture?Molar masses are MO2=32gmol,MN2=28gmol,MAr=40gmol. pressure rather than mass. Comment on why it may be easier to measure gases in CA Scuba diver wants to dive very deep and needs a special mixture of gasin his diving air tank. The gas consists of helium, nitrogenand oxygen. At a depth of 50 m, the pressure is about 600 kPa. In order for oxygen to dissolve in the blood of the diver, the partial pressure of oxygen must be at least 120 kPa. At the same time, the sum of the partial pressures of oxygen and nitrogen must be below 400 kPa, because higher oxygen/nitrogen pressures could lead to health problems for the diver. Therefore, the diver adds helium to make up the difference. If the tank has a volume of 30 liters and is at a temperature of 300K, how many moles of oxygen, nitrogen and helium are in the diver's air tank?
Step by Step Solution
★★★★★
3.35 Rating (173 Votes )
There are 3 Steps involved in it
Step: 1
9 P 120000 Pa ie 120 kPa V 102 m3 T 400 K using ideal ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


