Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If you can solve it and make sure its correct and in a clear writing i will be so thankful. The adiabatic exothermic irreversible gas-phase
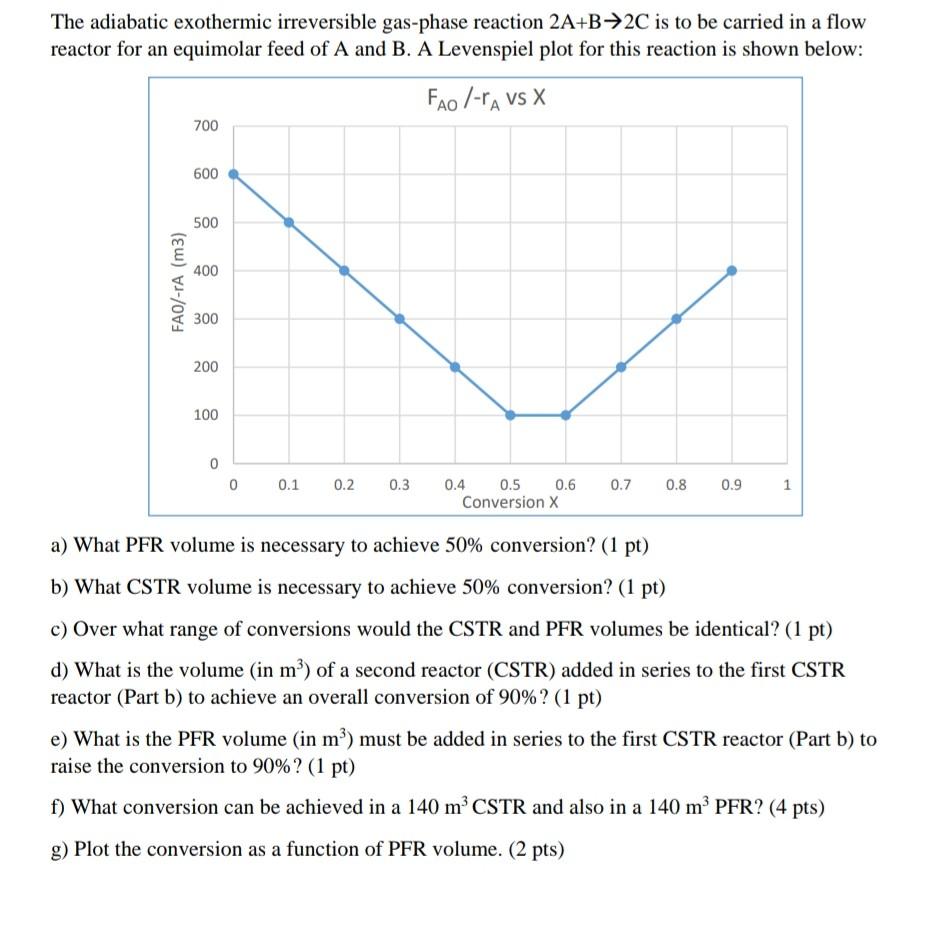
If you can solve it and make sure its correct and in a clear writing i will be so thankful.
The adiabatic exothermic irreversible gas-phase reaction 2A+B2C is to be carried in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown below: a) What PFR volume is necessary to achieve 50% conversion? (1 pt) b) What CSTR volume is necessary to achieve 50% conversion? (1 pt) c) Over what range of conversions would the CSTR and PFR volumes be identical? (1 pt) d) What is the volume (in m3 ) of a second reactor (CSTR) added in series to the first CSTR reactor (Part b) to achieve an overall conversion of 90% ? (1 pt) e) What is the PFR volume (in m3 ) must be added in series to the first CSTR reactor (Part b) to raise the conversion to 90%?(1pt) f) What conversion can be achieved in a 140m3 CSTR and also in a 140m3 PFR? (4 pts) g) Plot the conversion as a function of PFR volume. ( 2pts)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


