Answered step by step
Verified Expert Solution
Question
1 Approved Answer
if you could help me with all these questions itd be greatly appreciated! 12.14 There are three compounds with the molecular formula C2H2Br2. Two of
if you could help me with all these questions itd be greatly appreciated! 
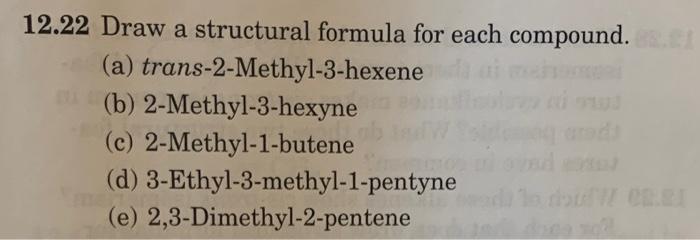
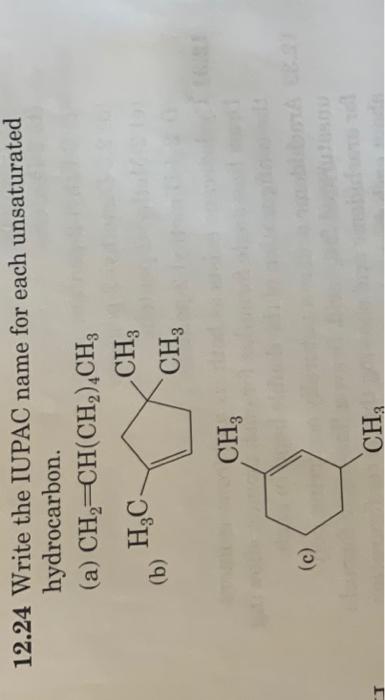



12.14 There are three compounds with the molecular formula C2H2Br2. Two of these are polar and have a dipole, and one has no dipole (it is not polar). Draw structural formulas for these three compounds and explain why two are polar and the third is not. 12.22 Draw a structural formula for each compound. (a) trans-2-Methyl-3-hexene (b) 2-Methyl-3-hexyne (c) 2-Methyl-1-butene (d) 3-Ethyl-3-methyl-1-pentyne (e) 2,3-Dimethyl-2-pentene 12.24 Write the IUPAC name for each unsaturated 12.26 Explain why each name is incorrect and then write a correct name. (a) 1-Methylpropene (b) 3-Pentene (c) 2-Methylcyclohexene (d) 3,3-Dimethylpentene (p) 4-Hpyyne (f) 2-Isopropyl-2-butene 12.30 Which of these alkenes shows cis-trans isomerism? For each that does, draw structural formulas for both isomers. (a) 1-Pentene (b) 2-Pentene (c) 3-Ethyl-2-pentene (d) 2,3-Dimethyl-2-pentene (e) 2-Methyl-2-pentene (f) 2,4-Dimethyl-2-pentene 2.32 Arachidonic acid is a naturally occurring polyunsaturated fatty acid. Draw a line-angle formula for arachidonic acid showing the cis configuration about each double bond. CH3(CH2)4(CH=CHCH2)4CH2CH2COOH 
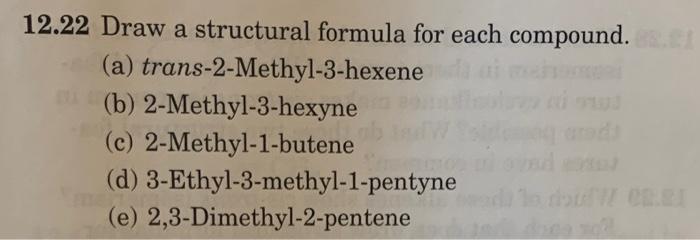




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


