Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ignore the pencil and red ink. Please show all steps needed 1. Complete a net ionic equation for the following proton-transfer reaction using curved arrows
Ignore the pencil and red ink. Please show all steps needed
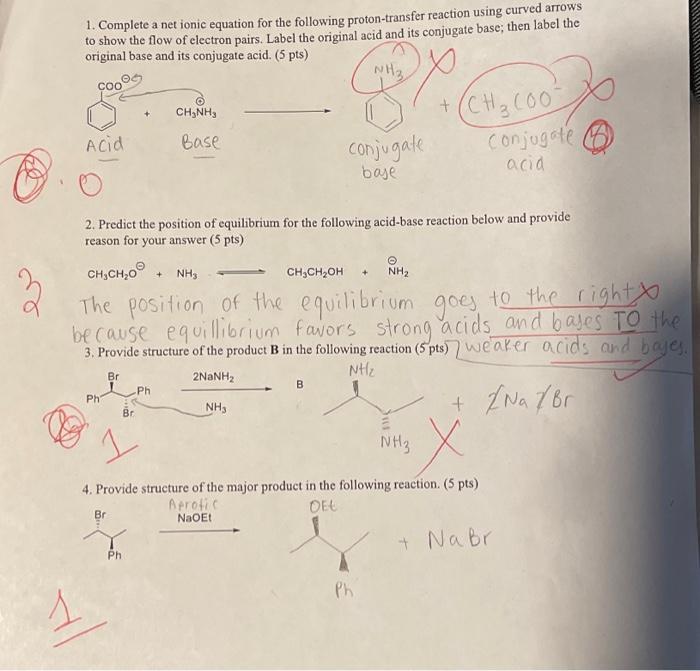

1. Complete a net ionic equation for the following proton-transfer reaction using curved arrows to show the flow of electron pairs. Label the original acid and its conjugate base; then label the original base and its conjugate acid. (5 pts) COO CH3NH3 Acid Base NH3 +(CH3COO conjugate baje Conjugate acid 2. Predict the position of equilibrium for the following acid-base reaction below and provide reason for your answer (5 pts) CHCH,O + NH3 CHCH2OH + NH The position of the equilibrium goes to the right because equilibrium favors strong acids and bases To the 3. Provide structure of the product B in the following reaction (5 pts) weaker acids and bayes. Br 2NaNH2 Ph Ph NH3 Br NHz + /Na/Br NH3 4. Provide structure of the major product in the following reaction. (5 pts) Br Ph Aprotic NaOEt DEE + Na br Ph
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


