Question: im having a hard time understanding why different people had different colors for our experiment. Project Experiment: Synthesis and Analysis of a Cobalt Amine Halide
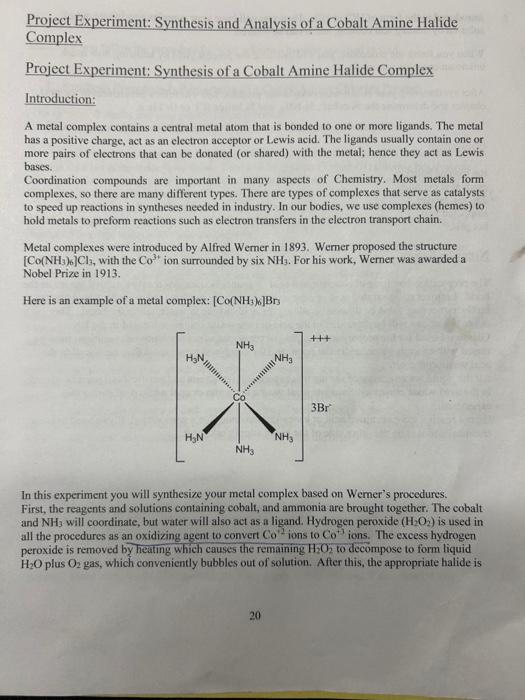








Project Experiment: Synthesis and Analysis of a Cobalt Amine Halide Complex Project Experiment: Synthesis of a Cobalt Amine Halide Complex Introduction: A metal complex contains a central metal atom that is bonded to one or more ligands. The metal has a positive charge, act as an electron acceptor or Lewis acid. The ligands usually contain one or more pairs of electrons that can be donated (or shared) with the metal; hence they act as Lewis bases. Coordination compounds are important in many aspects of Chemistry. Most metals form complexes, so there are many different types. There are types of complexes that serve as catalysts to speed up reactions in syntheses needed in industry. In our bodies, we use complexes (hemes) to hold metals to preform reactions such as electron transfers in the electron transport chain. Metal complexes were introduced by Alfred Wemer in 1893. Werner proposed the structure [Co(NH3)6]Cl3, with the Co3+ ion surrounded by six NH3. For his work, Werner was awarded a Nobel Prize in 1913. Here is an example of a metal complex: [Co(NH3)6]Br In this experiment you will synthesize your metal complex based on Wemer's procedures. First, the reagents and solutions containing cobalt, and ammonia are brought together. The cobalt and NH3 will coordinate, but water will also act as a ligand. Hydrogen peroxide (H2O2) is used in all the procedures as an oxidizing agent to convert Co2 ions to Co+1 ions. The excess hydrogen peroxide is removed by heating which causes the remaining H2O2 to decompose to form liquid H2OplusO2 gas, which conveniently bubbles out of solution. After this, the appropriate halide is introduced in the form of HCl or HBr. Then you will have your complex precipitate out of solution by heating and cooling, and it can be filtered. When you finish the procedure, you will calculate the percent yield by knowing how much unknown complex you made. After synthesizing your cobalt-ammonia-halide product, you will follow procedures in the next chapter and determine the % cobalt, %NH3, and % halide (Cl or Br) in it. Equipment top loading balance graduated cylinder 250mL beaker stoppered vial hot plate Notes on Safety The preparation/handling of concentrated acid or ammonia solutions must be carried out under the hood. Leftover concentrated acid or ammonium hydroxide solutions must not be added back to bottle, rather they must be diluted by adding the acid or base to water, under the hood. The dilutedsolutions will be discarded in the acid or base waste container located in the lab. Glassware used for the preparation/handling of concentrated acid or ammonia solutions must be rinsed with enough water, under the hood, and the combined rinses must then be placed in the acid waste container. All the liquid waste generated during this experiment (synthesis and purification) must be placed in the acid waste container. Students must wear a lab apron or coat while performing this experiment. Do not reach for concentrated acid or base over someone else's workstation. If you need to use a chemical and it is on the other side of the hood, simply ask for it. Procedures; Make sure you follow the procedure for your unknown number assigned to you! 1. Synthesis Procedure I: Preparation of a Cobalt-Amine-Chloride Product (The symbol, 0 marks stopping points if time is critical.) All of these steps will be done under the fume hood!! Remember Safety First!! Add 8 grams of ammonium chloride (NH4Cl) into 25mL deionized (DI) water. Boil this solution on a hot plate under fume hood. Then add 12 grams of cobalt (II) chloride hexahydrate (CoCl26H2O) to this solution when boiling. In your notebook, record the exact amount of CoCl26H2O that was added. Stir with a glass stirring rod to dissolve any solids. |Weigh out 2 grams of "activated" charcoal in powdered form. Add this to a 250mL beaker Pour the cooled solution of NH4Cl and CoCl26H2O into this 250mL beaker containing the charcoal. Cool this solution to room temperature. Add 25mL of ammonium hydroxide. Do this under the hood!! Place the beaker in an ice bath (water and ice) and stir with a glass stirring rod until cooled to 10C or lower. Use at thermometer for temperature check. Do not use the thermometer for stirring! While keeping the solution on ice, cautiously add 78mL of 30% hydromen peroxide. This needs to be done slowly, add the hydrogen peroxide in 5-8 drop portions with seconds in between and stirring between additions. The total time for adding all the H2O2 should takeas least 5 minutes but not exceed 15-20 minutes. CAUTION!! H2O2 IS A STRONG OXIDIZIVG AGENT AND MUST BE KEPT CLEAR OF EYES AND SKIN!! After all the hydrogen peroxide is added, heat the solution gradually to 6080C and maintain this temperature for about 30 minutes. If the contents start to get too hot, simply remove the beaker temporarily from the hot plate. Dark crystals may begin to form at the end of the heating After the 30 minutes, cool the beaker in an ice bath (10C). F Filter the cold mixture of dark crystals and liquid with a suction filter flask. Make sure you add water to the filter paper before filtering. Filter slowly! Do not wash the collected solid. This solid is a mixture of the insoluble black charcoal mixed with the orange product that you are making! 0 Purification (togor onercoal au of conpana) Dump the solid you collected in the Buchner funnel along with the filter paper into a 400 600mL beaker. Add 100mL of very hot (but not boiling) DI water to this beaker. Stir briefly and keep hot on a hot plate until the product crystals have dissolved (the black charcoal will not dissolve). Filter the very hot mixture (suction) immediately. Make sure filter flask is empty before starting!! Make sure you add DI water to the filter paper before filtering. Filter slowly! The 22 solution must be hot during the filtering process, since the charcoal will not go into solution, but your desired orange product will be soluble at a hot temperature. At this hot temperature, your solid will go through the filter paper and you can remove the charcoal. So, your desired orange product will be in the filter flask. This step is very important for best yields. Transfer all contents of the suction flask to a beaker and cool to room temperature. Use a little bit of DI water to make sure you get all onange solid form the suction flask. Add approximately 15mL of 12MHCl. Stir and cool the beaker to 10C in an ice bath. Filter (suction) to collect the orange crystals (your product!). Filter slowly! Wash the contents of the Buchner fumnel by pouring one approximately 10mL portion of ethyl alcohol over the precipitate while still on the filter. After the alcohol drains through the precipitate, wash again by pouring two approximately 10mL portions of acetone all over the solid on the filter. Let each drain before adding the next portion. 0 Dry in your drawer for one week. Next time you come to lab, you will weigh the product collected to the nearest 0.1 gram and add to vial. Heat the mixture with occasional stirring at 6080C for about 20 minutes. Cool the mixture in an ice bath to 10C. Filter the cold mixture with a suction filter flask. Make sure you add DI water to the filter paper before filtering. Filter slowly! Your desired magenta/purple product will be in the filter paper. Wash the product by pouring two approximately 5mL portions of ice water (DI water only.). Let each drain before adding the next portion. Wash the solid product with three approximately 5mL portions of acetone. Let the air continue to flow for 45 minutes. 0 Dry in your drawer for one week. Next time you come to lab, you will weigh the product collected to the nearest 0.1 gram and add to vial. 3. Synthesis Procedure III: Preparation of a Cobalt-A mine-Rromide Product (The symbol, O marks stopping points if time is critical.) All of these steps will be done under the fume hood!! Remember Safety First!! Mix 5 grams of cobalt carbonate (CoCO3) and 20mL of 9M(48%) hydrobromic acid ( HBr ) in a 250mL beaker. In your notebook, record the exact amoumt of CoCO3 that was added. Once frothing (foaming/bubbling) has stopped, add 15 mL deionized (DI) water. Stir briefly with a glass stirring rod. If the solution is not clear, filter the solution by gravity filtration, and discard the filter paper in the solid waste container along with any solid collected. Weigh out 2 grams of "activated" charcoal in powdered form. Add the charcoal to a different 250mL beaker. To this charcoal, add 25mL of ammonium hydroxide. Add the COCO3 solution into the beaker containing the charcoal. Place the beaker in an ice bath (water and ice) and stir with a glass stirring rod until cooled to 10C or lower. Use a thermometer for temperature check. Do not use the thermometer for stirring! While keeping the solution on ice, cautiously add 78mL of 30% hydrogen peroxide. This needs to be done slowly, add the hydrogen peroxide in 5-8 drop portions with 30 seconds in between and stirring between additions. The total time for adding all the H2O2 should take at least 5 minutes but not exceed 1520 minutes. CAUTION!! H2O2 IS A STRONG OXIDIZING AGENT AND MUST BE KEPT CLEAR OF EYES AND SKIN!! 9 After all the hydrogen peroxide is added, heat the solution to 6080C and maintain this temperature for 20 minutes. If the beaker contents start to get too hot, remove it temporarily from the hot plate, Stir occasionally with a glass stirring rod. Cool the mixture in an ice bath to approximately 5C to facilitate crystallization. Filter the cold mixture of dark crystals and liquid with a suction filter flask. Make sure you add DI water to the filter paper before filtering. Filter slowly! Do not wash the collected solid. This solid is a mixture of the insoluble black charcoal mixed with the orange product that you are making! 0 Heat the mixture with occasional stirring at 6080C for about 20 minutes. Cool the mixture in an ice bath to 10C. Filter the cold mixture with a suction filter flask. Make sure you add DI water to the filter paper before filtering. Filter slowly! Your desired magenta/purple product will be in the filter paper. Wash the product by pouring two approximately 5mL portions of ice water (DI water only.). Let each drain before adding the next portion. Wash the solid product with three approximately 5mL portions of acetone. Let the air continue to flow for 45 minutes. 0 Dry in your drawer for one week. Next time you come to lab, you will weigh the product collected to the nearest 0.1 gram and add to vial. 3. Synthesis Procedure III: Preparation of a Cobalt-A mine-Rromide Product (The symbol, O marks stopping points if time is critical.) All of these steps will be done under the fume hood!! Remember Safety First!! Mix 5 grams of cobalt carbonate (CoCO3) and 20mL of 9M(48%) hydrobromic acid ( HBr ) in a 250mL beaker. In your notebook, record the exact amoumt of CoCO3 that was added. Once frothing (foaming/bubbling) has stopped, add 15 mL deionized (DI) water. Stir briefly with a glass stirring rod. If the solution is not clear, filter the solution by gravity filtration, and discard the filter paper in the solid waste container along with any solid collected. Weigh out 2 grams of "activated" charcoal in powdered form. Add the charcoal to a different 250mL beaker. To this charcoal, add 25mL of ammonium hydroxide. Add the COCO3 solution into the beaker containing the charcoal. Place the beaker in an ice bath (water and ice) and stir with a glass stirring rod until cooled to 10C or lower. Use a thermometer for temperature check. Do not use the thermometer for stirring! While keeping the solution on ice, cautiously add 78mL of 30% hydrogen peroxide. This needs to be done slowly, add the hydrogen peroxide in 5-8 drop portions with 30 seconds in between and stirring between additions. The total time for adding all the H2O2 should take at least 5 minutes but not exceed 1520 minutes. CAUTION!! H2O2 IS A STRONG OXIDIZING AGENT AND MUST BE KEPT CLEAR OF EYES AND SKIN!! 9 After all the hydrogen peroxide is added, heat the solution to 6080C and maintain this temperature for 20 minutes. If the beaker contents start to get too hot, remove it temporarily from the hot plate, Stir occasionally with a glass stirring rod. Cool the mixture in an ice bath to approximately 5C to facilitate crystallization. Filter the cold mixture of dark crystals and liquid with a suction filter flask. Make sure you add DI water to the filter paper before filtering. Filter slowly! Do not wash the collected solid. This solid is a mixture of the insoluble black charcoal mixed with the orange product that you are making! 0 Purification Prepare a solution of 135mL DI water mixed with 3mL of concentrated 9MHBr. Heat until the solution is about to boil. Dump the solid you collected in the Buchner funnel along with the filter paper into a 400 600mL beaker. Add the near boiling solution of HBr to the filter paper in the beaker. Stir briefly and keep hot on a hot plate until the product crystals have dissolved (the black charcoal will not dissolve). Filter the very hot mixture (suction) immediately. Make sure filter flask is empty before starting!! Make sure you add DI water to the filter paper before filtering. Filter slowly! The solution must be hot during the filtering process, since the charcoal will not go into solution, but your desired orange product will be soluble at a hot temperature. At this hot temperature, your solid will go through the filter paper and you can remove the charcoal. So, your desired orange product will be in the filter flask. This step is very important for best yields. Transfer all contents of the suction flask to a beaker and cool to room temperature. Use a little bit of DI water to make sure you get all orange solid form the suction flask. Add approximately 10mL of concentrated (9M)HBr (hydrobromic acid). Cool to 10C or lower in an ice bath. Filter (suction) to collect the orange crystals (your product!). Make sure you add DI water to the filter paper before filtering. Filter slowly! Wash the contents of the Buichner funnel by pouring one approximately 10mL portion of ethyl alcohol over the precipitate while still on the filter. After the alcohol drains through the precipitate, wash again by pouring two approximately 10mL portions of acetone all over the solid on the filter. Let each drain before adding the next portion. 9 Dry in your drawer for one week. Next time you come to lab, you will weigh the product collected to the nearest 0.1 gram and add to vial. 4. Synthesis Procedure IV: Preparation of Cobalt-Amine-Bromide Product (The symbol, 0 marks stopping points if time is critical.) All of these steps will be done under the fume hood!! Remember Safety First!! Weigh 8 grams of cobalt (II) bromide hexahydrate (CoBr26H2O) and add to a 250mL beaker. In your notebook, record the exact amount of CoBr2 - 6H2O that was added. To this beaker, add 20mL of deionized (DI) water. Warm to approximately 50C to dissolve. In another 250mL beaker, mix 15 grams of NH NBr4 with 30mL of concentrated aqueous ammonia. Stir vigorously with a glass stirring rod. The NHaBr will not completely dissolve. Slowly add the CoBr2 - 6H2O solution from the first beaker to the NH4Br solution. Place the beaker in an ice bath (water and ice) and stir with a glass stirring rod until cooled to 10C or lower. Use a thermometer for temperature check. Do not use the thermometer for stirring! While keeping the solution on ice, eautiously add 7-8 mL of 30% hydrogen peroxide. This needs to be done slowly, add the hydrogen peroxide in 58 drop portions with 30 seconds in between and stirring between additions. The total time for adding all the H2O2 should take at least 5 minutes but not exceed 1520 minutes, CAUTION!! H2O2 IS A STRONG OXIDIZING AGENT AND MUST BE KEPT CLEAR OF EYES AND SKIN!! After all the H2O2 has been added, heat the solution to 90100C and evaporate under the hood to a slurry about 2/3 of the original volume when heating began. Continued stirring will reduce the time for this operation (2040 minutes). If the beaker contents start to get too hot, remove it temporarily from the hot plate until it cools somewhat. 9 Once evaporated to roughly two-thirds of the original volume, cool in an ice bath to room temperature or below. Prepare 4.5MHBr by adding 25mL of 9M(48%)HBr to 25mL of DI water. Add approximately 50mL of the newly prepared 4.5MHBr to the recently boiled solution. Stir briefly. Heat the mixture with occasional stirring at 6070C for about 60 minutes. Cool in an ice bath to 10C or lower. Filter the cold mixture with a suction filter flask. Make sure you add DI water to the filter paper before filtering. Filter slowly! Your desired magenta/purple product will be in the filter paper. Wash the product by pouring two approximately 5mL portions of ice water (DI water only.). Let each drain before adding the next portion. Wash the solid product with three approximately 5mL portions of acetone. Let the air continue to flow for 4-5 minutes. 0 Dry in your drawer for one week. Next time you come to lab, you will weigh the product collected to the nearest 0.1 gram and add to vial. 5. Synthesis Procedure V: Preparation of a Cobalt-Amine-Bromide Product (The symbol, 0 marks stopping points if time is critical.) All of these steps will be done under the fume hood!! Remember Safety First!! Slowly add 4 grams of cobalt carbonate (CoCO3) to 15mL of 9M(48%) hydrobromic acid (HBr) in a 250mL beaker. In your notebook, record the exact amount of CoCO3 that was added. Once frothing (foaming/bubbling) has stopped, add 10mL deionized (DI) water. Stir briefly. Filter the solution by gravity filtration, and discard the filter paper in the solid waste container along with any solid collected. Prepare a solution containing 7 grams NH4Br dissolved in 35mL of ammonium hydroxide in a 250mL beaker. The NH4Br will not completely dissolve. Add the CoCOs solution into the beaker containing the NH4Br. Place the beaker in an ice bath (water and ice) and stir with a glass stirring rod until cooled to 10C or lower. Use a thermometer for temperature check. Do not use the thermometer for stirring! While keeping the solution on ice, cautiously add 7.8mL of 30% hydrogen peroxide. This needs to be done slowly, add the hydrogen peroxide in 58 drop portions with 30 seconds in between and stirring between additions. The total time for adding all the H2O2 should take at least 5 minutes but not exceed 15-20 minutes. CAUTION!! H2O2 IS A STRONG OXIDIZING AGENT AND MUST BE KEPT CLEAR OF EYES AND SKINI! 1. Does everyone in the lab have the same color for product? Y(N) What other colors are there? -Red 2. Why do you suppose some have different colors? Some peopie had different colors due to the different complex structore of the and different Cobat Amine Halide. Number of amine iigand
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


