Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I'm not sure on how to find standard deviation and percent error. Thank you. Trial 1 Use of a Pipet Trial 2 Trial 3 Mass
I'm not sure on how to find standard deviation and percent error. Thank you. 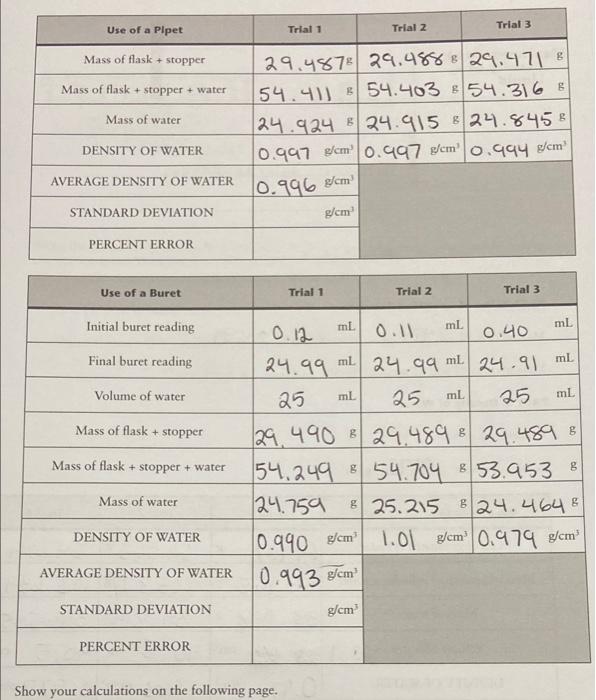
Trial 1 Use of a Pipet Trial 2 Trial 3 Mass of flask + stopper Mass of flask + stopper + water 29.487 29.488829.471 8 54.411 8 54.403 854.316 g 24.924 8 24.915 8 24.8458 0.997 g/cm 0.997 gem 0.994 g/cm! Mass of water DENSITY OF WATER AVERAGE DENSITY OF WATER 0.996 cm STANDARD DEVIATION g/cm PERCENT ERROR Use of a Buret Trial 1 Trial 2 Trial 3 Initial buret reading mb ml mL Final buret reading ml ml Volume of water ml ml ml Mass of flask + stopper 8 0.12 0.11 0.40 24.99 ml 24.99 24.91 25 25 25 29.490 829.489 & 29.489 8 54,249 8 54,704 8 53.953 8 8 24.759 8 25.215 8 24.464 8 8 B g/ 1.01 g/cm 0.974 g/cm Mass of flask + stopper + water 8 Mass of water DENSITY OF WATER 0.990 cm 10.993 g/cm AVERAGE DENSITY OF WATER STANDARD DEVIATION g/cm PERCENT ERROR Show your calculations on the following page 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


