Question
Imagine two solutions with the same concentration and the same boiling point, but one has benzene as the solvent and the other has carbon
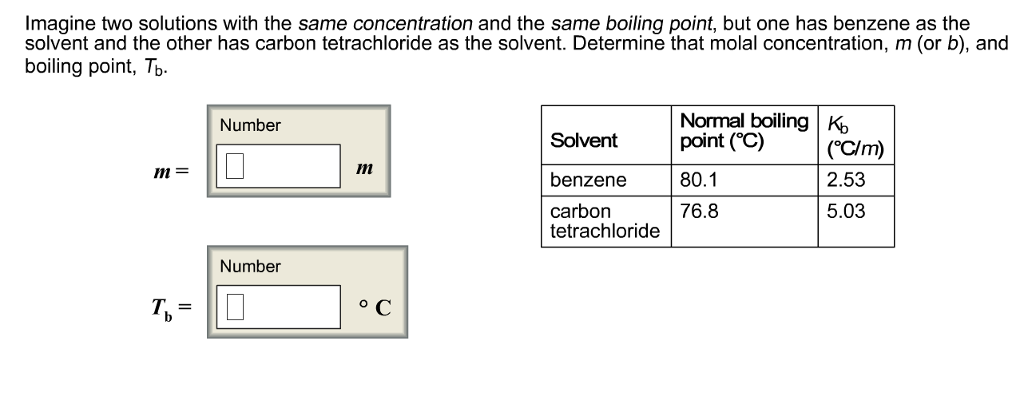
Imagine two solutions with the same concentration and the same boiling point, but one has benzene as the solvent and the other has carbon tetrachloride as the solvent. Determine that molal concentration, m (or b), and boiling point, Tb. m= T= Number Number m C Solvent benzene carbon tetrachloride Normal boiling K point (C) 80.1 76.8 (C/m) 2.53 5.03
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Answer Tb mkb ATb Change in boiling point Boiling point after addition of soluteTp Normal bo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: David Young, Shane Stadler
10th edition
1118486897, 978-1118836873, 1118836871, 978-1118899205, 1118899202, 978-1118486894
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



