Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Imagine you are given a metal cylinder and asked to determine whether it is made of gold, lead, copper, or iron. The densities of
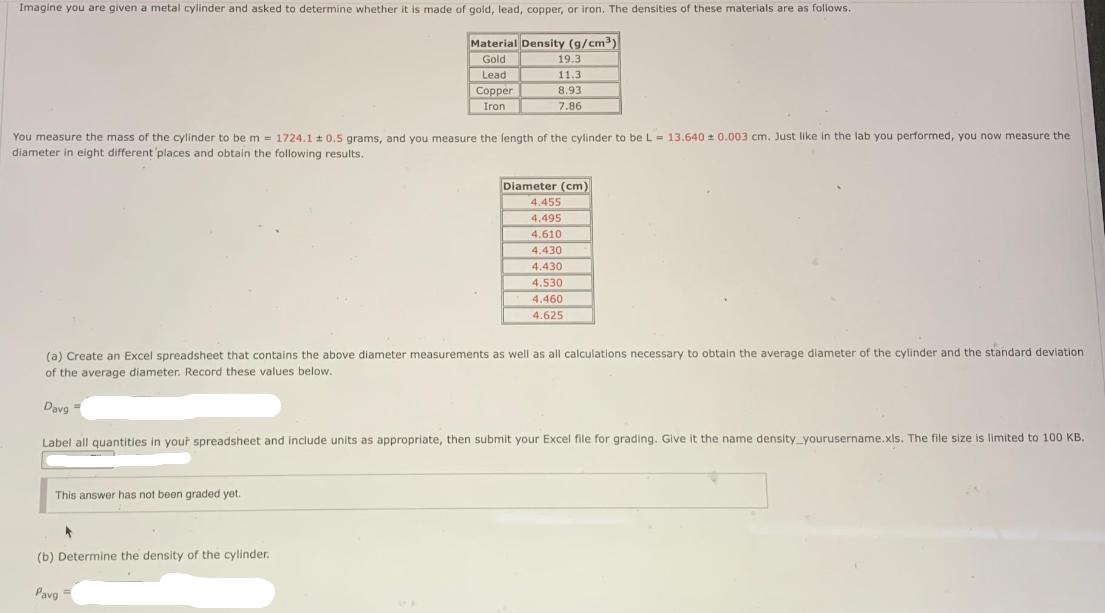
Imagine you are given a metal cylinder and asked to determine whether it is made of gold, lead, copper, or iron. The densities of these materials are as follows. Material Density (g/cm) Gold Lead Copper Iron You measure the mass of the cylinder to be m = 1724.1 0.5 grams, and you measure the length of the cylinder to be L = 13.640 0.003 cm, Just like in the lab you performed, you now measure the diameter in eight different places and obtain the following results. 19.3 11.3 8.93 7.86 This answer has not been graded yet. (a) Create an Excel spreadsheet that contains the above diameter measurements as well as all calculations necessary to obtain the average diameter of the cylinder and the standard deviation of the average diameter. Record these values below. (b) Determine the density of the cylinder. Diameter (cm) 4.455 4.495 4.610 Davg= Label all quantities in your spreadsheet and include units as appropriate, then submit your Excel file for grading. Give it the name density_yourusername.xls. The file size is limited to 100 KB. Pavg= 4.430 4.430 4.530 4.460 4.625
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Determining the Density of the Cylinder To determine the density of the cylinder we can follow these ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


