Answered step by step
Verified Expert Solution
Question
1 Approved Answer
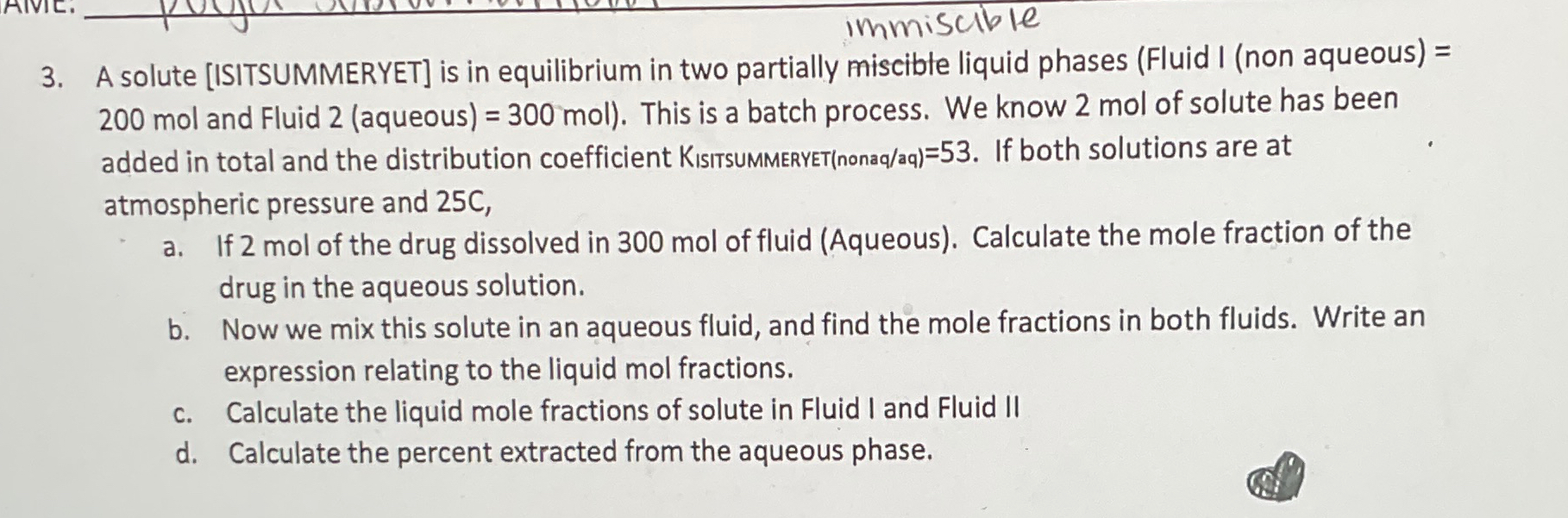
immiscible 3 . A solute [ ISITSUMMERYET ] is in equilibrium in two partially Immiscible liquid phases ( Fluid I ( non aqueous ) =
immiscible
A solute ISITSUMMERYET is in equilibrium in two partially Immiscible liquid phases Fluid I non aqueousmol and Fluid aqueousmol This is a batch process. We know mol of solute has been added in total and the distribution coefficient KISITTSUMMERYETnonaqaq If both solutions are at atmospheric pressure and
a If mol of the drug dissolved in mol of fluid Aqueous Calculate the mole fraction of the drug in the aqueous solution.
b Now we mix this solute in an aqueous fluid, and find the mole fractions in both fluids. Write an expression relating to the liquid mol fractions.
c Calculate the liquid mole fractions of solute in Fluid I and Fluid II
d Calculate the percent extracted from the aqueous phase.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


