Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In a binary laminar gas mixture, equimolar counter transfers of A and B occur at 1 bar pressure. The molar concentrations of A at point
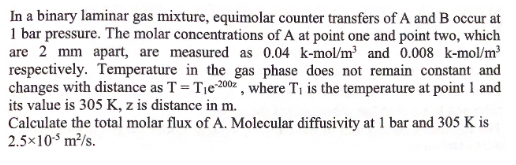 In a binary laminar gas mixture, equimolar counter transfers of A and B occur at 1 bar pressure. The molar concentrations of A at point one and point two, which are 2mm apart, are measured as 0.04kmol/m3 and 0.008kmol/m3 respectively. Temperature in the gas phase does not remain constant and changes with distance as T=T1e2002, where T1 is the temperature at point 1 and its value is 305K,z is distance in m. Calculate the total molar flux of A. Molecular diffusivity at 1 bar and 305K is 2.5105m2/s
In a binary laminar gas mixture, equimolar counter transfers of A and B occur at 1 bar pressure. The molar concentrations of A at point one and point two, which are 2mm apart, are measured as 0.04kmol/m3 and 0.008kmol/m3 respectively. Temperature in the gas phase does not remain constant and changes with distance as T=T1e2002, where T1 is the temperature at point 1 and its value is 305K,z is distance in m. Calculate the total molar flux of A. Molecular diffusivity at 1 bar and 305K is 2.5105m2/s Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


