Question
In a Calorimetry experiment, a 20.0g metal ball is heated to 150 Cand dropped into a perfectly insulating container with 200 g of water
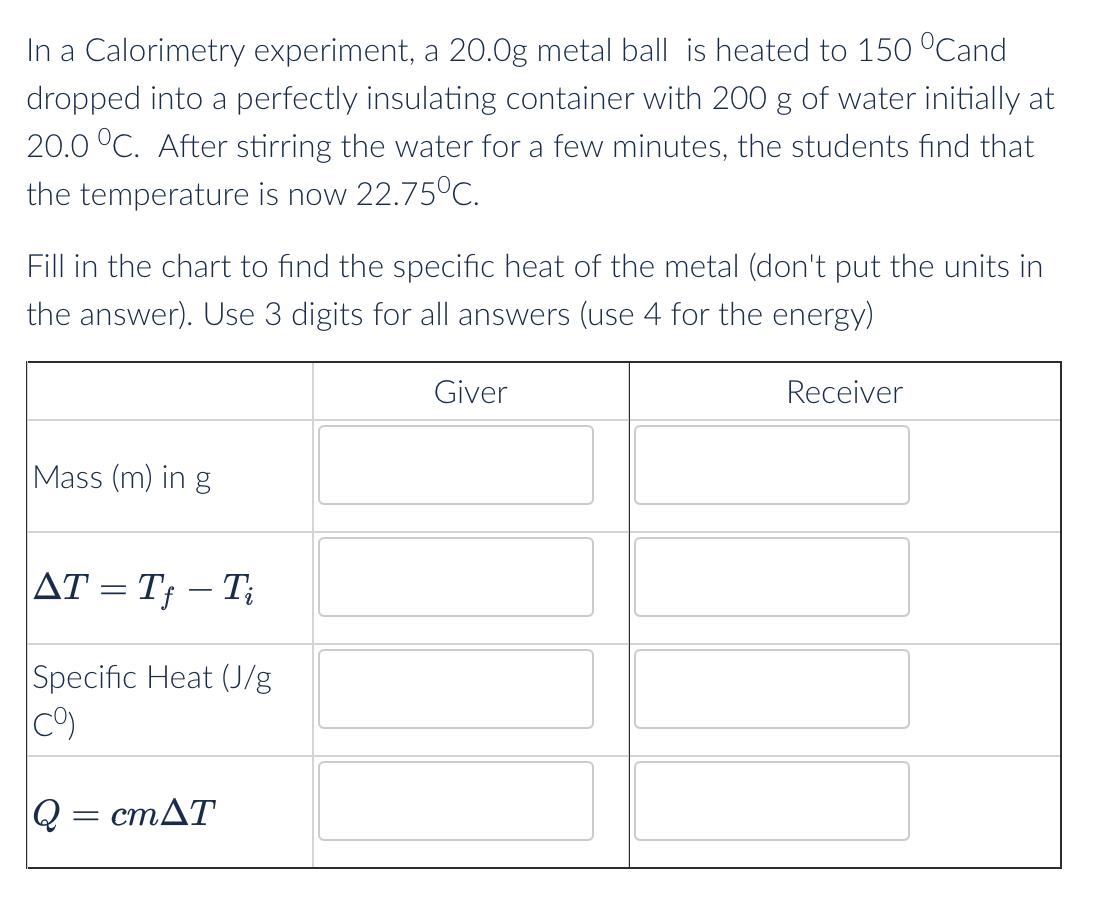
In a Calorimetry experiment, a 20.0g metal ball is heated to 150 Cand dropped into a perfectly insulating container with 200 g of water initially at 20.0 C. After stirring the water for a few minutes, the students find that the temperature is now 22.75C. Fill in the chart to find the specific heat of the metal (don't put the units in the answer). Use 3 digits for all answers (use 4 for the energy) Mass (m) in g AT = Tf Ti - Specific Heat (J/g C) Q = cmAT Giver Receiver
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics
Authors: Jerry D. Wilson, Anthony J. Buffa, Bo Lou
7th edition
9780321571113, 321601831, 978-0321601834
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



