Question
In a reactor of known volume (V), which is equal to 15 L, it is desired to carry out the reaction in the liquid phase
In a reactor of known volume (V), which is equal to 15 L, it is desired to carry out the reaction in the liquid phase at a constant temperature. The feed is introduced with a volumetric flow rate of 4 L/min. To characterize the behavior of the reactor beforehand, suspecting that it behaves like a tank-type reactor with dead volume, an impulse tracer test is carried out using the above-mentioned volume. From this, the variation of the tracer concentration is measured over time at the reactor's exit. The results obtained are shown below. Calculate the average residence time and the dead and active volumes of the reactor. Consider the reaction as A + B producing C.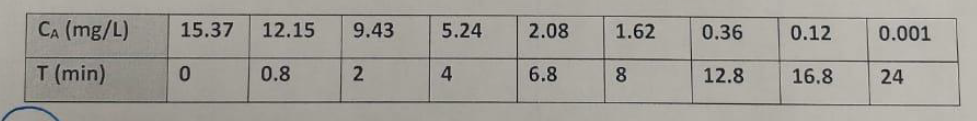
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


