Question
In a study of the gas phase decomposition of hydrogen peroxide at 400 C HO(g) +HO(g) + O(9) the concentration of HO was followed
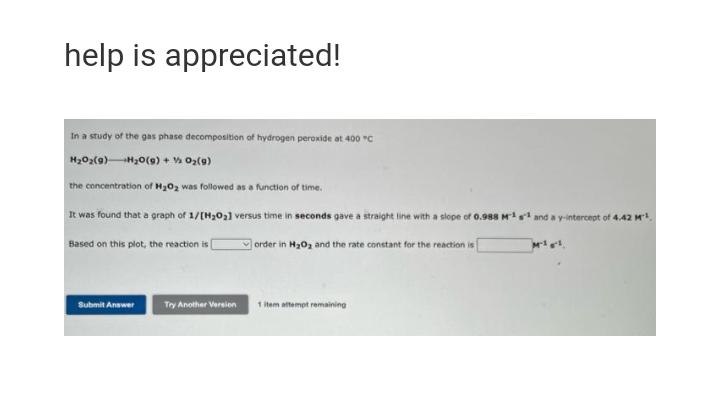
In a study of the gas phase decomposition of hydrogen peroxide at 400 "C HO(g) +HO(g) + O(9) the concentration of HO was followed as a function of time. It was found that a graph of 1/[H0] versus time in seconds gave a straight line with a slope of 0.988 M1s and a y-intercept of 4.42 M Based on this plot, the reaction is order in HO and the rate constant for the reaction is Submit Answer Try Another Version 1 item attempt remaining
Step by Step Solution
3.56 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics Learning From Data
Authors: Roxy Peck
1st Edition
495553263, 978-1285966083, 1285966082, 978-0495553267
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



