Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In a sulfuric acid plant, sulfur is burned in the presence of excess oxygen to produce sulfur dioxide, (SO,) which in turn is further
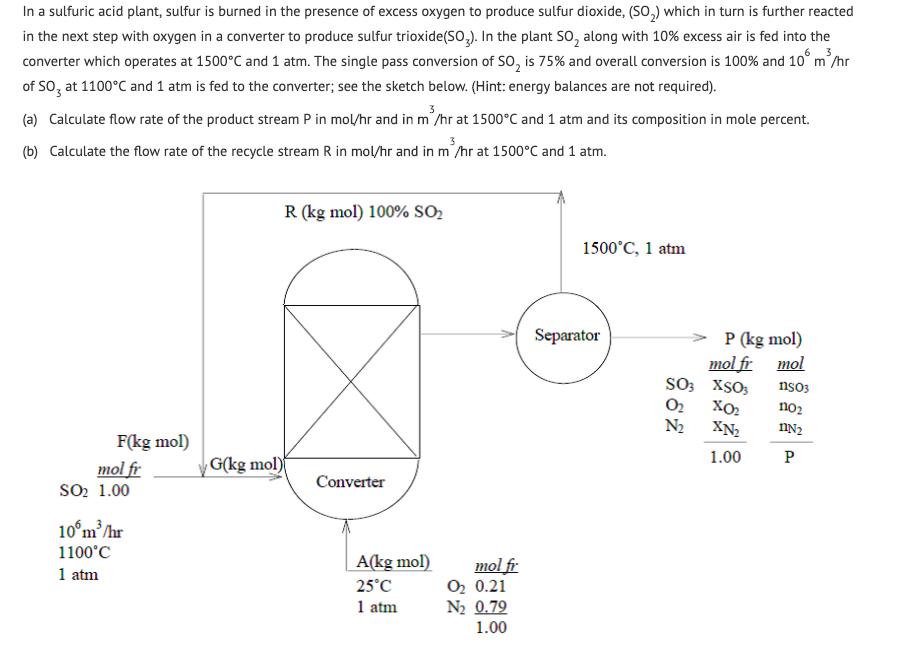
In a sulfuric acid plant, sulfur is burned in the presence of excess oxygen to produce sulfur dioxide, (SO,) which in turn is further reacted in the next step with oxygen in a converter to produce sulfur trioxide(SO,). In the plant SO, along with 10% excess air is fed into the converter which operates at 1500C and 1 atm. The single pass conversion of SO, is 75% and overall conversion is 100% and 10 m'/hr of SO, at 1100C and 1 atm is fed to the converter; see the sketch below. (Hint: energy balances are not required). (a) Calculate flow rate of the product stream P in mol/hr and in m'/hr at 1500C and 1 atm and its composition in mole percent. (b) Calculate the flow rate of the recycle stream R in mol/hr and in m'/hr at 1500C and 1 atm. R (kg mol) 100% SO2 1500C, 1 atm Separator P (kg mol) mol fr mol SO3 XSO3 O2 N2 nso3 no2 XN2 F(kg mol) 1.00 P G(kg mol) mol fr Soo 1.00 Converter 10m/hr 1100C A(kg mol) mol fr O2 0.21 N2 0.79 1.00 1 atm 25C 1 atm
Step by Step Solution
★★★★★
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Solution By considering material balance around the SO 2 feeding point F R G Given F 10 6 m 3 hr By ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


