Question
In an experiment, a perfect gas is placed in a vessel with a massless piston. A massless wire is attached to this piston. When an
In an experiment, a perfect gas is placed in a vessel with a massless piston. A massless wire is attached to this piston. When an external pressure of 2 atm is applied to this wire, the gas is compressed from 4.90 liters to 2.45 liters. Subsequently, when the external pressure is increased to 2.50 atm, the gas is compressed from 2.45 liters to 1.96 liters.
In another independent experiment with the same initial conditions, an external
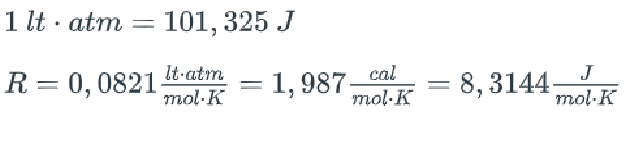
pressure of 2.50 atm is applied to the perfect gas, resulting in a reduction in volume from 4.90 liters to 1.96 liters in a single step.
Since the final temperature of the gas is the same in both experiments, calculate the heat flow difference in Joules between the two-stage compression process and the single-stage compression process?
1ltatm=101,325JR=0,0821molKltatm=1,987molKcal=8,3144molKJ 1ltatm=101,325JR=0,0821molKltatm=1,987molKcal=8,3144molKJStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


