Question
In an experiment to determine A and equilibrium constant of weak electrolyte by applying Ostwald dilution law, the following equation was obtained by plotting
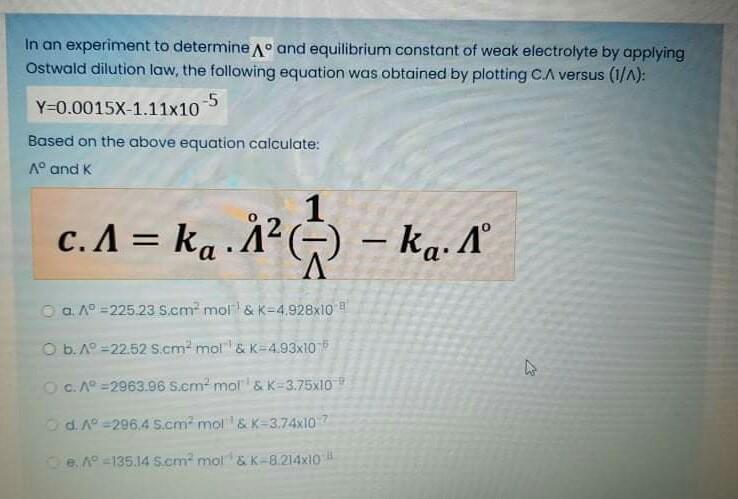
In an experiment to determine A and equilibrium constant of weak electrolyte by applying Ostwald dilution law, the following equation was obtained by plotting C.A versus (1/^): Y-0.0015X-1.11x10-5 Based on the above equation calculate: A and K c. A = ka. ka. 1 () a. A =225.23 S.cm mol & K-4.928x10 O b. A -22.52 S.cm mol & K-4.93x10 O c. A =2963.96 S.cm mol & K-3.75x10 d. A=296.4 S.cm mol & K-3.74x10 e. A=135.14 S.cm mol & K-8.214x10 - ka. A
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Answer From the line with 6 Thus A given equation plot of en Vs ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For Engineers And Scientists
Authors: William Navidi
3rd Edition
73376345, 978-0077417581, 77417585, 73376337, 978-0073376332
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



