Answered step by step
Verified Expert Solution
Question
1 Approved Answer
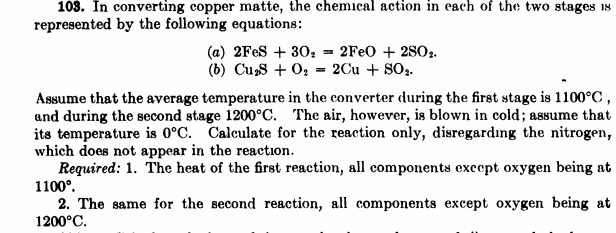
In converting copper matte, the chemical action in each of the two stages is represented by the following equations: ( a ) 2 FeS +
In converting copper matte, the chemical action in each of the two stages is represented by the following equations:
aFeSFeO
b
Assume that the average temperature in the converter during the first stage is and during the second stage The air, however, is blown in cold; assume that its temperature is Calculate for the reaction only, disregarding the nitrogen, which does not appear in the reaction.
Required: The heat of the first reaction, all components except oxygen being at
The same for the second reaction, all components except oxygen being at b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


