Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In equimolar counter diffusion for every mole of one component diffusing in one direction there is, by definition, one mole of the other component


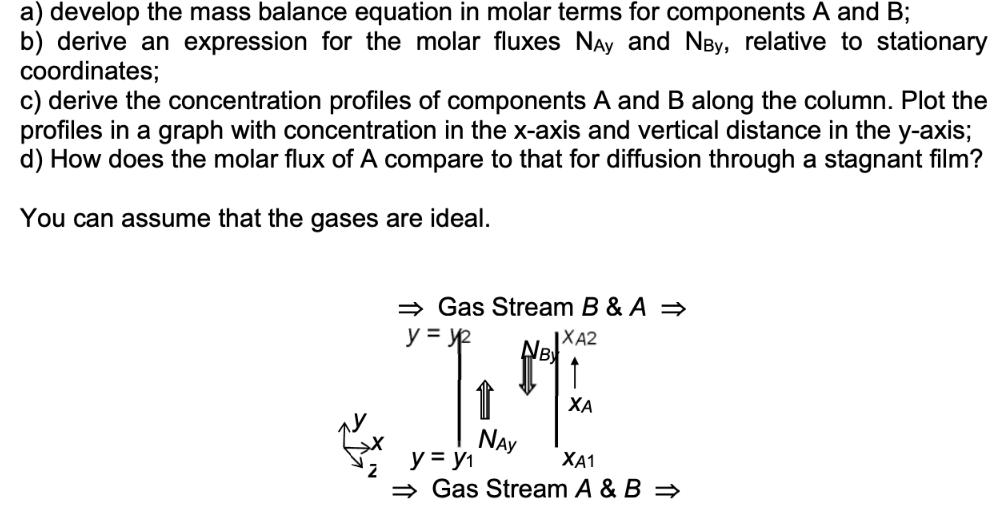
In equimolar counter diffusion for every mole of one component diffusing in one direction there is, by definition, one mole of the other component diffusing in the opposite direction. In this problem we will consider mass transfer by diffusion in the vertical column of constant cross-section (the shape of the cross-section is not significant) shown in Figure below. The column is open at both ends. Inside the column there are two components A and B. Gas streams containing both components A and B flow across each of the two open ends of the column at the top and the bottom. The concentration of A in the lower stream is greater than the concentration of A in the upper stream giving rise to a concentration difference, which drives the mass transfer of A from the bottom to the top of the column. For equimolar counter diffusion, we have NAY = -NBy in the column, where NAY and NBy are the molar fluxes of the components A and B respectively in the vertical direction in the column, relative to stationary coordinates. The geometry has been chosen so that there is mass transfer in one direction only, the y- direction and Cartesian coordinates can be used. Use a differential control volume inside the column and a) develop the mass balance equation in molar terms for components A and B; b) derive an expression for the molar fluxes Nay and NBy, relative to stationary coordinates; c) derive the concentration profiles of components A and B along the column. Plot the profiles in a graph with concentration in the x-axis and vertical distance in the y-axis; d) How does the molar flux of A compare to that for diffusion through a stagnant film? You can assume that the gases are ideal. Gas Stream B & A y = y XA2 N & y = y NAY XA XA1 Gas Stream A & B
Step by Step Solution
★★★★★
3.54 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
a Lets consider a differential control volume inside the column of height dy with crosssectional are...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


